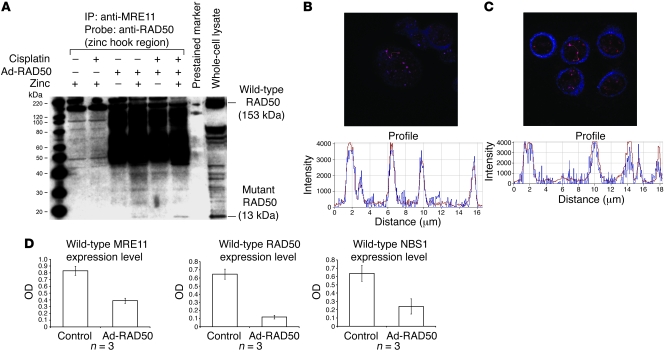
Figure 3. Mutant RAD50 protein interacts with endogenous RAD50 and downregulates the MRN complex.
(A) Anti-MRE11 antibody coimmunoprecipitated wild-type RAD50 in all cells, suggesting a direct interaction between these proteins. Mutant RAD50 was only coimmunoprecipitated in infected cells treated with zinc, indicating that the interaction between mutant RAD50 and the wild-type MRN complex is mediated by zinc-dependent dimerization of RAD50 hook domains and not direct interactions between mutant RAD50 and MRE11. All lanes shown were run simultaneously on a single gel as contiguous lanes. (B) Multiple MRN nuclear foci are seen at sites of DNA damage in JHU012 cells treated with Ad-RAD50 and cisplatin. Original magnification, ×63. There is spatial overlap of the signals corresponding to wild-type RAD50 (blue) and wild-type MRE11 (red), highly suggestive of a binding interaction between these proteins. (C) Nuclear foci from JHU012 cells treated with Ad-RAD50 and cisplatin (original magnification, ×63) demonstrate spatial overlap of the wild-type (red) and mutant RAD50 (blue) proteins, further suggesting direct binding between these proteins. (D) Downregulation of wild-type MRE11 (P < 0.005), RAD50 (P < 0.001), and NBS1 proteins (P < 0.04) is seen after infection of JHU012 cells with Ad-RAD50 relative to noninfected controls. (Mean optical density of Western blot bands ± SEM is shown.) This is attributable to the demonstrated dimerization between mutant and wild-type RAD50 and inability of the mutant protein to directly bind MRE11. Consequently, only a single MRE112/RAD502 heterotetramer can form around the wild-type RAD50 with no complimentary heterotetramer assembling around mutant RAD50.