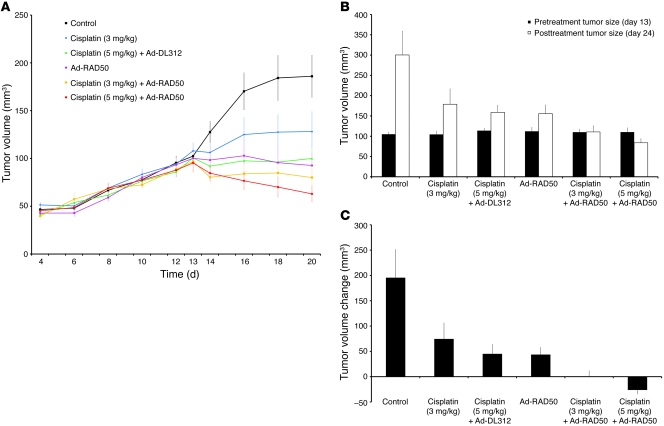
Figure 7. Ad-RAD50 chemosensitizes JHU012 human head and neck cancer to cisplatin in vivo.
(A) Externally measured tumor volume ± SEM from the time of initial tumor palpability on day 4, to treatment initiation on day 13, and through the treatment period to day 20. (B) Mean internally measured tumor volume ± SEM immediately prior to treatment on day 13 and at the time of animal sacrifice on day 24. (C) Mean internally measured change in tumor volume ± SEM. During the 11 day period between treatment initiation and animal sacrifice, a single administration of Ad-RAD50 combined with a single intraperitoneal dose of cisplatin, at either 3 mg/kg or 5 mg/kg, was able to significantly enhance the therapeutic efficacy of cisplatin (3 mg/kg or 5 mg/kg) monotherapy. For Ad-RAD50 with cisplatin (3 mg/kg), P < 0.001 vs. control; P < 0.05 vs. cisplatin (3 mg/kg) and cisplatin (5 mg/kg) with DL312; and P < 0.05 vs. Ad-RAD50 alone. For Ad-RAD50 with cisplatin (5 mg/kg), P < 0.001 vs. control, cisplatin (3 mg/kg), cisplatin (5 mg/kg) with DL312, and Ad-RAD50 alone.