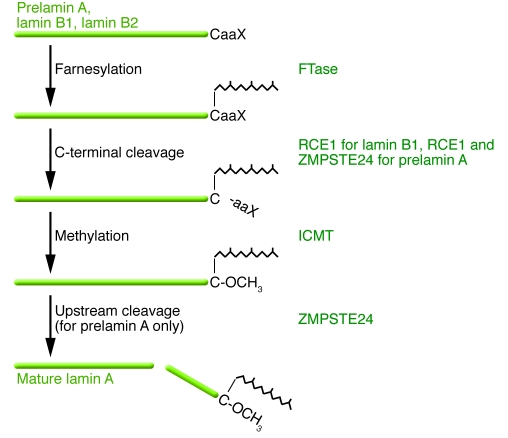
Figure 2. Schematic diagram outlining the posttranslational processing of nuclear lamins.
Prelamin A and B-type lamins undergo 3 sequential posttranslational processing steps. First, the cysteine of the carboxyl-terminal CaaX motif is farnesylated by protein FTase. Second, the –aaX is clipped off. For prelamin A, this is likely a redundant activity of RCE1 and ZMPSTE24. Third, the newly exposed carboxyl-terminal farnesylcysteine is methylated by isoprenylcysteine carboxyl methyltransferase (ICMT). Prelamin A undergoes another step in which the carboxyl-terminal 15 amino acids, including the farnesylcysteine methyl ester, are clipped off by ZMPSTE24 and degraded, generating mature lamin A. In the setting of ZMPSTE24 deficiency, the final endoproteolytic cleavage does not occur, leading to the accumulation of a farnesylated and methylated prelamin A. ZMPSTE24 deficiency causes a severe progeroid disorder, RD. In HGPS, an alternative splicing event results in a 50–amino acid deletion in prelamin A, removing the site for the final endoproteolytic cleavage step. Thus, mature lamin A cannot be produced, and cells accumulate a mutant prelamin A that terminates with a farnesylcysteine α-methyl ester.