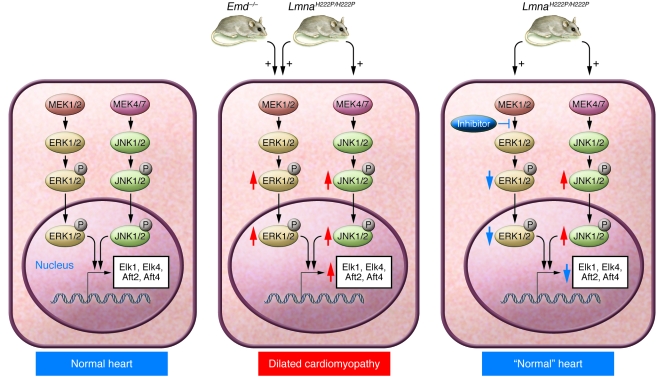
Figure 3. Studies from LmnaH222P/H222P knockin mice and Emd-knockout mice suggest that activation of ERK and/or JNK underlies the development of cardiomyopathy.
Cardiomyocytes in normal hearts of wild-type mice exhibit detectable ERK and JNK activation, as judged by low levels of expression of downstream transcription factors such as Elk1, Elk4, Aft2, and Aft4 (left panel). Both ERK and JNK signaling are increased in hearts from mice harboring the H222P point mutation in Lmna, whereas ERK is activated in hearts of Emd-knockout mice (red arrows; middle panel). Phosphorylation and nuclear translocation of ERK and JNK modulate gene expression, leading to dilated cardiomyopathy (middle panel). Currently, it is unclear how alterations in A-type lamins or the loss of emerin lead to the activation of ERK and/or JNK. Studies in LmnaH222P/H222P mice have shown that pharmacological inhibition of MEK, the kinase that phosphorylates ERK, can prevent the development of cardiomyopathy at 16 weeks of age (right panel).