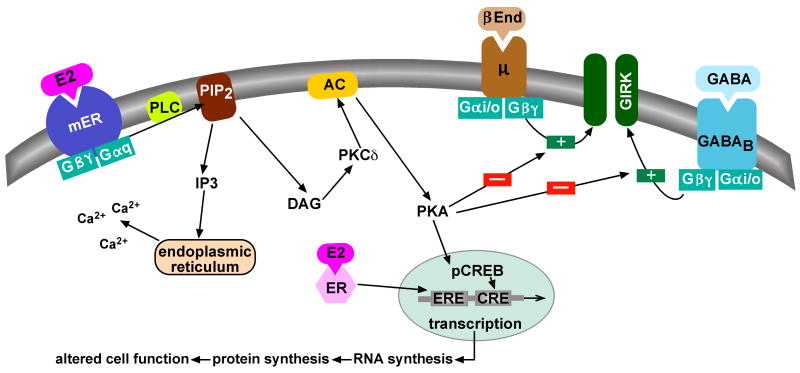
Figure 1. Cellular models of membrane-initiated signaling pathways of E2.
Besides the classical ER-mediated gene activation via an estrogen response element (ERE), E2 can activate a membrane-associated ER (mER) that is Gαq -coupled to activation of phospholipase C that catalyzes the hydrolysis of membrane-bound phosphatidylinositol 4,5-biphosphate (PIP2) to inositol 1,4,5 triphosphate (IP3) and diacylglycerol (DAG). Calcium is released from intracellular stores (endoplasmic reticulum) by IP3, and DAG activates protein kinase Cδ (PKCδ). Through phosphorylation, adenylyl cyclase (AC) activity is upregulated by PKC. The generation of cAMP activates PKA, which can rapidly uncouple GABAB and μ-opioid (μ) receptors from their effector system through phosphorylation of a downstream effector molecule (e.g., G protein-coupled, inwardly rectifying K+ (GIRK) channel). The mER-mediated modulation of kinase pathways reduces the capacity of neuromodulators such as GABA and β-endorphin (β-End) to inhibit POMC neuronal excitability. Finally, mER-mediated activation of PKA can lead to phosphorylation of cAMP-response element binding protein (pCREB), which can then alter gene transcription through its interaction with a CREB response element (CRE).