Fig. 3.
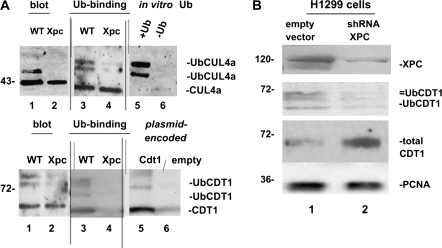
(A) CUL4A and CDT1 cell cycle checkpoint proteins in cultured wild-type and Xpc−/− bone marrow. We wanted to determine if the presence or absence of XPC would alter DNA damage signalling. Immunoblots were conducted using 50 μg of total cell lysates (lanes 1 and 2, upper and lower panels). Equal amounts of cellular proteins, 5 mg, were then affinity purified on a ubiquitin-binding resin and immunoblotting of bound proteins was conducted. The higher molecular weight ubiquitinated forms of CUL4A (upper panel) and CDT1 (lower panel) clearly differ between the two genotypes (lanes 3 and 4 of upper and lower panels). The data suggest a defect in CUL4A and CDT1 ubiquitin modification in Xpc−/− mice. To help identify ubiquitinated CUL4A and CDT1, we used in vitro ubiquitin-conjugated proteins. Omission of ubiquitin from the reaction shows that mainly ubiquitinated CUL4A is detected by the CUL4A antibody (lanes 5 and 6, upper panel). Plasmid-encoded CDT1 protein was also used as a marker (lanes 5 and 6, lower panel). (B) Knocking down XPC in H1299 cells alters the ubiquitin modification of CDT1. We used transiently transfected H1299 cells expressing an shRNA to XPC to test if the results shown in panel (A) were due directly to loss of XPC. Although Xpc was not completely silenced, it was clearly decreased by the shRNA. Higher molecular weight ubiquitinated forms of CDT1 were largely absent where XPC was knocked down, consistent with a role for XPC in DNA damage signalling to the CDT1 cell cycle checkpoint protein.