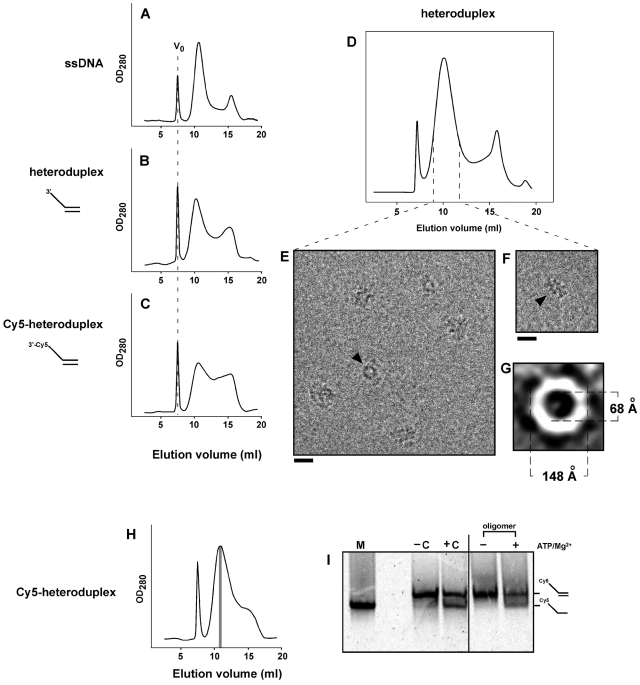
Figure 5. Rep68 forms an active helicase oligomer in the presence of ss-dsDNA heteroduplex.
Rep68 (1 mg/ml, 16.6 µM) was incubated with 2.8 µM ssDNA (A), 2.8 µM heteroduplex (B), or 2.8 µM Cy5-heteroduplex (C) on ice for 30 min. After centrifugation, 50-µL of sample was chromatographed on a Superose 6 HR 10/30 column. Elution of Rep68 oligomers was followed by UV detection at 280 nm. V0 position is represented as dashed line in Rep68 panels. (D) Size-exclusion chromatography of Rep-heteroduplex in buffer A. Central part of the high-MW peak (dashed lines) was concentrated, and mixed with n-octyl β-D-glucopyranoside just before cryo-EM analysis. (E and F) Representative images of the heteroduplex-Rep68 oligomer; ring-shaped and elongated particles are shown by arrowheads. Bars correspond to 20 nm. (G) A representative class average of end views is shown; internal and external dimensions of the ring are shown in Angstroms. (H) Rep68 (16.6 µM) was incubated with 2.1 µM Cy5-heteroduplex, and chromatographed as above. Three hundred-µL fractions were collected, and fraction corresponding to the central part of the Cy5-hetroduplex/Rep68 oligomer (in gray) was 4-fold concentrated. (I) Concentrated oligomer was incubated in the absence (−) or presence (+) of 1 mM MgCl2/1 mM ATP for 30 min at 37°C. Helicase reactions were analyzed on 16% polyacrylamide gels combined with Cy5 detection. −C and +C correspond to the negative (substrate alone) and positive (substrate plus Rep68) controls respectively, which were incubated in the presence of 1 mM MgCl2/1 mM ATP. M, Cy5-ssDNA marker (oligo JM-37). At right, positions for Cy5-heteroduplex and Cy5-ssDNA are indicated.