Abstract
Purpose
To analyze patterns of failure in patients with head and neck cutaneous squamous cell carcinoma (HNCSCC) and clinical/radiological evidence of perineural invasion (CPNI), in order to define neural clinical target volume (CTV) for treatment planning.
Methods
Patients treated with 3D conformal or intensity modulated radiotherapy (IMRT) for HNCSCC with CPNI were included in the study. A retrospective review of the clinical charts, radiotherapy (RT) plans and radiological studies has been conducted.
Results
Eleven consecutive patients with HNCSCCs with CPNI were treated from 2000 through 2007. Most patients received multiple surgical procedures and RT courses. The most prevalent failure pattern was along cranial nerves (CNs), and multiple CNs were ultimately involved in the majority of cases. In all cases the involved CNs at recurrence were the main nerves innervating the primary tumor sites, as well as their major communicating nerves. We have found several distinct patterns of disease spread along specific CNs depending on the skin regions harboring the primary tumors, including multiple branches of CN V and VII. These patterns and the pertinent anatomy are detailed in the paper.
Conclusions
Predictable disease spread patterns along cranial nerves supplying the primary tumor sites were found in this study. Awareness of these patterns, as well as knowledge of the relevant cranial nerve anatomy, should be the basis for CTV definition and delineation for RT treatment planning.
Keywords: Skin cancer, Head and neck, IMRT, Target definition
INTRODUCTION
Perineural invasion (PNI) is identified in approximately 3.7% of head and neck cutaneous squamous cell carcinoma (HNCSCC) cases 1. In addition to being associated with increased risk of regional and distant metastases 1,2, PNI is most prominently associated with increased risk of local recurrences, including recurrences in both the skin and in cranial nerves (CNs). Together, these local recurrences account for the majority of failures following definitive treatment 3,4.
In cases with clinical or radiological evidence for CN involvement the term clinical PNI (CPNI) is used 2,5, while for cases in which PNI is incidentally found on pathologic examination, the term microscopic PNI (MPNI) is used.
Radiotherapy (RT) has a central role in the treatment of HNCSCC with CPNI, either as adjuvant treatment following surgical resection, or as definitive treatment for unresectable disease. The need to irradiate cranial nerves close to organs at risk (e.g. optic structures, brain and inner ear) accounts for the high rate (>30%) of complications associated with RT in these cases 4,6. Using conformal or intensity modulated RT may improve the therapeutic index of treatment. For purposes of highly conformal RT, the definition and delineation of the clinical target volumes (CTVs) for regions at risk of subclinical disease is of utmost importance. A systematic analysis of recurrence patterns supporting guidelines for CTV delineation is lacking.
We have encountered several patients with MPNI or CPNI with treatment failures following conformal RT or IMRT. These failures were due to CPNI progression that seemed to follow distinct patterns. The aim of this paper is to analyze the patterns of disease progression and treatment failures, compare these failures with the targets chosen for irradiation, and review the relevant anatomy and pertinent literature, in an attempt to outline guidelines for target volume delineation in the treatment of HNCSCC with CPNI.
METHODS
Patients were retrospectively identified through the data-base of the Radiation Oncology department. Inclusion criteria were:
Histologically proven HNCSCC.
Diagnosis of CPNI- biopsy proven CN involvement or clinical CN dysfunction in nerves distributed to the primary skin tumor site. Magnetic resonance imaging (MRI) findings without histological evidence for PNI or CN dysfunction were not sufficient to establish the diagnosis of CPNI.
Treatment with RT for the index cancer at any disease stage.
CPNI resulting from the primary skin tumor (as opposed to CN involvement by an advanced nodal disease).
The data was collected retrospectively from the medical charts. RT plans and imaging studies were systematically reviewed.
Kaplan-Meier analysis was used for calculation of progression free survival and overall survival.
RESULTS
The clinical features, treatments and outcome of patients are summarized in Tables 1 and 2. Patient numbers throughout the paper relate to these Tables.
Table 1. Radiotherapy (RT,) patterns of failure, and outcome.
RT after surgical resection is designated “adjuvant” and RT to an uninvolved site is designated “elective”. In all other cases RT was delivered for gross disease.
| Target and intent in 1st RT course | Site of failure after 1st RT course | Target and intent in 2nd RT course | Site of failure after 2nd RT course | Survival after the diagnosis of CPNI (months) | current status | |
|---|---|---|---|---|---|---|
| 1 | nerves* | treated nerves | nerves | N/A† | 66m | alive |
| 2 | skin-adjuvant* | nerves | nerves | contiguous nerves | 17m | alive |
| 3 | skin and nerves | skin and contiguous nerves | nerves-palliative | 60m | dead | |
| 4 | skin-adjuvant | nerves | nerves | contiguous nerves | 80m | alive |
| 5 | skin-adjuvant* | skin and nerves | skin and nerves-adjuvant | N/A† | 4m | alive |
| 6 | skin-adjuvant* | skin and nerves | skin and nerves-adjuvant, nodes-elective | skin and contiguous nerves | 22m | dead |
| 7 | skin and nerves – adjuvant, nodes-elective | contiguous nerves | nerves | treated and contiguous nerves | 13m | alive |
| 8 | skin and nerves-adjuvant, nodes-elective | N/A† | 39m | alive | ||
| 9 | Skin and nerves. nodes-elective | N/A† | 10m | alive | ||
| 10 | skin-adjuvant | nerves contiguous | nerves-palliative | radiotherapy discontinued after 12.5 Gy | 2m | dead |
| 11A†† | Nerves. Nodes-elective. | nerves | nerves | N/A | 28m | alive |
| 11B | Skin. Nerve and nodes-elective. | contiguous nerve |
RT was delivered in another facility.
N/A=not applicable (no evidence for disease recurrence)
Patient 11 was synchronously diagnosed with bilateral tumors with clinical perineural invasion (CPNI) which were considered as two separate cases.
Table 2. Details of perineural invasion, radiotherapy (RT) for cranial nerves (CNs) and patterns of failure in CNs.
These results summarize clinical (C), radiological(R), and surgical (S) data. Involvement of trigeminal ganglion, auriculotemporal nerve (ATN) and greater superficial petrosal nerve (GSPN) was always identified radiologically.
| Site of primary | Neural target in first RT course to nerves | Site of failure following first RT course to nerves | Site of failure following second RT course to nerves | CN involvement, sequence of events | |
|---|---|---|---|---|---|
| 1 | primary not identified | CN V, VII and cavernous sinus | Treated nerves | V2-3(C), VII(C), trigeminal ganglion | |
| 2 | temple | V2-3 and cavernous sinus | V1 and CN VII | temporal branch of facial nerve(S), V1-3(C), trigeminal ganglion, GSPN, VII(C), III, IV, VI(C) | |
| 3 | forehead | V1 and cavernous sinus | V3 | V1(C), trigeminal ganglion, V2-3(C), VII(C), III, IV, VI (C) | |
| 4 | medial cheek | V2-3 and cavernous sinus | V1 and CN VII | V2-3(C), GSPN, VII(C), V1 (C), trigeminal ganglion, III, IV, VI (C) | |
| 5 | lateral eyebrow | V1 | V1(S) | ||
| 6 | preauricular | CN VII | ATN and V3 | VII(C), V3(C,R)+ATN | |
| 7 | ear | CN VII | ATN and V3 | V2, inferior alveolar verve, cysternal segment of CN V | VII(C), ATN+ V3(R), trigeminal ganglion, V2(R) |
| 8 | cheek | CN VII, ATN, V3 | VII(S), ATN, V3(S) | ||
| 9 | cheek | CN VII, ATN, V3 | VII(C), ATN, V3(R) | ||
| 10 | nasal ala | CN VII and V | VII(C), V(C) | ||
| 11A | primary not identified | left V3 | left CN VII | left V3(C), VII(C) | |
| 11B | ear | right CN VII (excluding the cochlea) | right tympanic portion of CN VII | right VII(C) |
Clinical Findings
Initial presentation of skin cancer
Eleven patients, treated with RT between the years 2000–2007 for twelve HNCSCCs with CPNI, fulfilled the inclusion criteria. The median age at the time of diagnosis was 67 years (range 53–89 years), and there was male predominance (male/female ratio 8/3).
Three patients had received chronic immunosuppressive treatment; two for kidney transplant (patients 10 and 11) and one for Wegener’s granulomatosis (patient 7). The majority of patients were initially treated in other facilities for the primary skin cancer; therefore, information was lacking regarding the clinical characteristics of the primary skin tumor (e.g. size, depth). Six tumors were moderately or poorly differentiated and three were well differentiated. MPNI was histologically identified in five primary tumors and three cutaneous recurrences.
Details of CPNI
The typical presentation of CPNI was focal CN V or VII dysfunction. Common symptoms included numbness, pain or paresthesia in the distribution of the affected division of CN V and focal facial palsy. In three cases the first evidence for CPNI was nerve involvement found during surgery (Table 2).
The pattern of primary CN involvement reflected the innervation of primary skin tumor sites and the proximity of this site to major nerve trunks; periauricular tumors in patients 6, 7, 8, 9 and 11b infiltrated CN VII, supraorbital tumors in patients 3 and 5 infiltrated V1, and a medial cheek tumor in patient 4 infiltrated V2. Patient 2 had a tumor of the temporal area that involved both V2–3 and CN VII at an early stage.
The majority of cases (excluding patient 8) presented with a single nerve involvement; subsequently, in ten cases, involvement of additional nerves was observed after less than one year.
The mechanism of secondary CNs involvement could be elucidated in the following cases (Table 2):
In four patients (6, 7, 8, 9) the disease spread from CN VII through the auriculotemporal nerve (ATN) to V3 (Figures 1a–b).
In two patients (2 and 4) with clinical involvement of V2 and CN VII, involvement of the greater superficial petrosal nerve (GSPN) was demonstrated by MRI. The GSPN most likely allowed progression of disease between V2 and CN VII (Figures 2a–b)
One patient (patient 1) presented with involvement of peripheral branches of both CN V and CN VII without evidence for involvement of major communicating nerves. Subsequently tumor involvement progressed proximally along these nerves to the base of skull.
In four patients (2, 3, 4, 7) the disease spread from a major branch of CN V to involve additional CN V branches, most likely through the cavernous sinus (Figures 2c–e).
In four patients (2, 3, 4, and 5) disease spread through V1 or V2 to the orbit (Figures 2e–f). In three of these patients extraocular motor nerves (EOMN; i.e. CN III, IV and VI) were secondarily involved either through involvement of the orbital apex or through involvement of the cavernous sinus.
In two patients (2 and 9) concentric growth of tumor along peripheral nerve branches was noted (Figures 1b, 2a, 2f), likely due to antegrade disease progression.
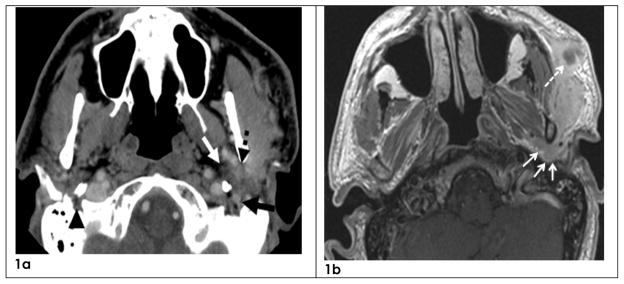
Figure 1.
Cranial nerves VII, V3 and Auriculotemporal nerve (ATN)
1a-Axial CT from patient 9 showing an abnormal V3 (white arrow), ATN (dashed black arrow) and CN VII (black arrow) on the left side. Normal CN VII is shown on the right side (black arrowhead).
1b-Axial MRI image from patient 9 showing abnormal ATN (arrows) and subcutaneous mass (dashed arrow). The subcutaneous mass most likely represents growth of disease along peripheral branches of CN VII.
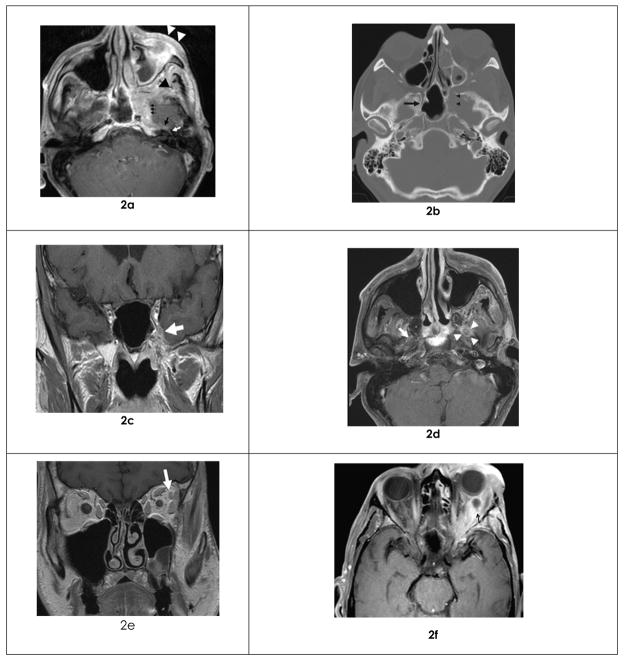
Figure 2.
Multiple cranial nerves involvement in one patient (patient 2)
2a-Tumor involving the Pterygopalatine ganglion (of CN V) in the Pterygopalatine fossa (black arrowhead), Pterygoid canal (small arrows), Greater superficial petrosal nerve (GSPN, black arrow) and CN VII (white arrow) on axial MRI image. The GSPN inside the Pterygoid canal is termed “nerve of pterygoid canal”.
Involvement of subcutaneous tissue in V2 distribution as a result of antegrade tomor progression is shown as well (white arrowheads).
2b-Abnormal expansion and destruction of the Pterygoid canal is shown by axial CT (arrowheads). The normal right canal is indicated (arrows).
2c- Abnormal V2 at the cavernous sinus on coronal MRI image (arrow).
2d-Abnormal V3 on axial MRI image (arrowheads). The normal right V3 is indicated for comparison (arrow).
2e-Involvement of the lacrimal branch of V1 on coronal MRI image (arrow).
2f-MRI image dated 10 months after image 2e, showing extensive orbital involvement on axial MRI image.
Utility of imaging studies
Eleven of 12 patients were evaluated with MRI before therapy (excluding patient 8). In all cases, clinical signs or symptoms prompted an MRI and preceded the radiological evaluation. In only one patient (patient 6) was the MRI negative despite clinical symptoms of, partial, unilateral facial palsy.. However, six months later MRI showed involvement of V3, ATN and CN VII.
MRI established the diagnosis of CPNI in six patients, showed progression of disease along nerves in three asymptomatic patients, and revealed the full extent of disease and was useful for treatment planning in six cases with CPNI.
Treatment
Surgical interventions
In ten cases multiple and extensive surgical procedures were performed for the primary skin tumor. Parotidectomy was performed in five cases, and resection of facial bones was performed in five cases (temporal, zygomatic or nasal bone). Multiple local recurrences were documented in seven cases and Mohs’ surgery was adequate for removal of the skin tumor in only one case.
Resection of branches or the main trunk of CN VII was performed in four cases (patients 2, 6, 7, and 8). Patient 5 underwent resection of the supraorbital nerve (SON) through the orbital apex.
RT to the primary skin tumor
Five patients received a course of adjuvant electron beam RT to the primary tumor bed in the skin. The median RT dose in these cases was 60 Gy (range 45–64 Gy).
RT to involved nerves
Excluding the first course of RT in patient 1, all RT courses for CPNI were performed at our department. The intention of therapy varied (Table 1): adjuvant treatment after resection of a nerve, definitive treatment for gross disease or palliation of symptoms. Four patients were treated twice with RT for CPNI, to adjacent, mostly non-overlapping sites. In case 11b elective RT to an uninvolved nerve (CN VII) was delivered along with definitive RT to an auricular tumor.
In seven RT courses the skin was treated along with the nerve. RT to the skin was omitted in the other cases that did not have skin recurrence and had undergone previous RT to the skin.
In total, fourteen courses of conformal RT for CPNI were delivered, seven of which constituted of IMRT. Any gross abnormality (based on imaging findings) was defined as gross tumor volume (GTV). Microscopically involved nerves were defined as high risk clinical target volume (CTV). The CTVs always included the uninvolved portion of the involved nerve beyond the GTV through the base of skull (typically, the cavernous sinus for involvement of CN V, and the stylomastoid foramen and the inner ear for involvement of CN VII) (Figure 3). However, elective RT to uninvolved nerves was delivered only in case 11B. The skin target included GTV or resected tumor bed with wide margins.
Figure 3.
Examples for clinical target volume (CTV) delineation (nerves at risk are shown in yellow, CTVs in red)
3a-CTV encompassing the supraorbital nerve (V1) which courses in the roof of the orbit (superior to the superior rectus muscle), major branches of V1, the skin innervated by V1 and the cavernous sinus.
3b-CTV encompassing the infraorbital nerve (V2) in the infraorbital canal, the skin innervated by V2 and the cavernous sinus
3c, d-CTV encompassing preauricular skin, CN VII, the Auriculotemporal nerve (ATN), V3 and cavernous sinus. The volume named “cochlea” (3c) contains both a target (CN VII), and the organ at risk. The course of the Greater superficial petrosal nerve (GSPN) is indicated by broken line.
In six cases radiation fields included the regional lymph nodes in the neck. The median dose of RT to neural GTVs in definitive cases was 68 Gy (range 66–70 Gy). The dose of RT to neural CTVs ranged between 50 Gy and 66 Gy. In two palliative cases doses of 40–50 Gy were prescribed to neural GTVs. In nine cases RT was combined with chemotherapy (Carboplatin-three patients, Carboplatin+Pactitaxel-two patients, Docetaxel-one patient) or Cetuximab (three patients).
Treatment results and patterns of failure (Table 1)
Five patients who initially received a course of adjuvant electron beam RT to the skin only, ultimately experienced disease recurrence, which involved CPNI in all cases; in two patients, skin recurrence was also observed.
Eight patients had disease recurrence following RT for CPNI in contiguous, previously uninvolved nerves. This was the primary pattern of failure in five cases; in two patients neural progression was combined with skin involvement and in one patient an in-field neural failure was also observed. No nodal or distant failure was observed in any patient.
Overall outcome
Patient 10 declined continuation of RT after 12.5 Gy (in the second RT course) and died one month later. Treatment interruption for two days, was necessary in patient 2, due to nausea, vomiting and dehydration, after a dose of 16 Gy, The other patients completed their treatment without any interruption or any prohibitive acute toxicity.
Patients 3 and 4 were treated with RT to the orbit. In patient 3 the irradiated eye was blind prior to the treatment due to retinal detachment, and patient 4 lost vision in the irradiated eye 14 months after completion of RT. This patient had extensive orbital disease and the mean dose to the optic nerve was 68 Gy. We did not observe any other major late side effects. The median progression free survival of patients receiving RT for CPNI was 5 months and the median overall survival was 60 months.
DISCUSSION
The patterns of disease progression and treatment failure observed in this series prompt discussion on several key issues pertinent to target definition in HNCSCC with PNI:
-
Inclusion of uninvolved nerves in the CTV for adjuvant treatment
in patients with MPNI
in patients with CPNI
Extent of the CTV for an involved CN
Optimization of RT planning.
1A) Inclusion of uninvolved nerves in the CTV for adjuvant treatment in patients with MPNI
While extensive RT is obviously justified in patients with CPNI, elective CN RT (ECNR) is not common practice in cases of MPNI. We encountered five patients who were treated with adjuvant RT to the skin only for MPNI. They all progressed to CPNI and salvage RT was unsuccessful; indeed, even as primary treatment, RT for CPNI was associated with low rates of local control in our series, similar to reports by others 7,8. However, this series includes patients who were referred to a tertiary center because their disease was exceptionally aggressive or due to CN involvement. In addition, three patients were under immunosuppressive treatment. Therefore, the disease course in the patients included in this series does not necessarily represent the natural history of all cases with MPNI.
Mendenhal et. al. published the largest series of RT for HNCSCC with PNI and proposed criteria for inclusion of nerves in the target in patients with MPNI 2,9. They recommend ECNR in cases with extensive MPNI, involved surgical margins, or involvement of named nerve branch. Mendenhall et. al. suggested that the target should include the involved nerve with a margin of several centimeters in cases with extensive MPNI, while in cases with positive margins or involvement of a named nerve branch, the entire course of the nerve to the base of skull would be included.
In some cases, the involved nerve is not identified during surgery. Based on patterns of disease spread found in this study and in other series 8, 10, 11, Preauricular tumors (i.e. lateral to the lateral canthus) are likely to involve CN VII first, and tumors in the mid-face (i.e. medial to the lateral canthus) are likely to involve CN V first; however, both CN V and CN VII are always at risk, and the CTV should be customized to account for the individual risk for each nerve.
1B) Inclusion of uninvolved nerves in the CTV for adjuvant treatment in patients with CPNI
A tumor with proven involvement of one nerve is likely to involve additional nerves. We encountered several patterns of spread which correlate with those described in the literature:
CN V +VII (Figures 4a–b)
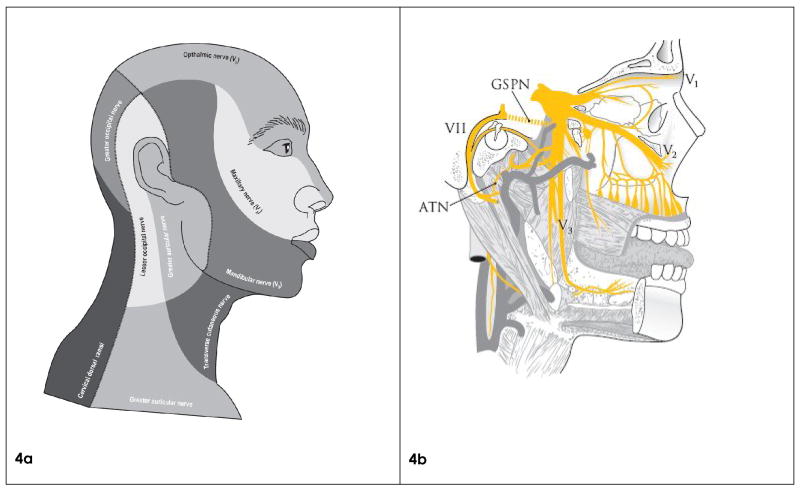
Figure 4.
The major nerves innervating facial skin and musculature
4a. Facial skin regions according to distribution of the various sensory nerves. Note that skin tumors at any facial site can spread via both the respective sensory nerve and also via cranial nerve VII if the underlying muscle is invaded by tumor.
4b. Diagram of CN V and VII and their connections.
Note the Auriculotemporal nerve (ATN) and Greater superficial petrosal nerve (GSPN) connecting CN VII with V3 and V2, respectively, allowing tumor spread between these nerves.
In this study involvement of both CN V and VII was highly prevalent and was present in eleven of twelve cases.
Three different mechanisms may account for this pattern of disease progression:
Independent risk due to dual innervation of facial skin and musculature by CN V and VII, respectively.
Communications between terminal branches of CN V and CN VII.
Two major nerves connect CN V and VII: ATN 5, 12, which connects V3 with CN VII as it exits through the stylomastoid foramen, and the GSPN 5, 13, connecting the geniculate ganglion of CN VII and the pterygopalatine ganglion of V2 (Figure 4b). The ATN is peripheral, while the GSPN is intracranial; therefore, the ATN may be involved earlier in the course of disease, when curative treatment is more likely.
Our experience as well as others’ 8, 10, 11, 17 confirms that all three mechanisms play a role in the natural history of HNCSCC with CPNI. Therefore, for tumors involving CN VII, the ATN and V3 are at risk and for tumors involving V2 the GSPN and CN VII are at risk.
Due to the risk of direct involvement of both CN V and VII by the primary tumor (as in patient 1), the branches of these nerves that are distributed to the primary tumor are always at risk and should be included in the CTV.
Multiple branches of CN V
In our study multiple branches of CN V were involved in five patients.
The underlying mechanisms are similar to those described above: direct involvement of more than a single trunk, or spread between two trunks through either peripheral or major nerve communications 14. The entire CN V might be affected secondary to involvement of the cavernous sinus followed by antegrade extension (Figure 4b). Therefore, for tumors involving V1, 2, or 3, the other branches of CN V are at risk.
CN V and EOMN
In our study three patients had CN V and EOMN involvement.
Disease involving V1 or V2 can enter the orbit and subsequently progress to involve CN III, IV and VI 8, 10, 11, 15–17, resulting in orbital-apex or cavernous sinus syndrome. This syndrome manifests as ophthalmoplegia, ptosis, pain, and visual loss in advanced cases. Therefore, for tumors involving V1 or V2, EOMN are at risk.
A literature search was conducted for series of HNCSCC and PNI which report the rate of CN involvement. The results are summarized in Table 3. These data likely underestimate the true incidence of CN involvement due to their retrospective nature and lack of accurate imaging in some series; however, they are consistent with the patterns observed in our study.
Table 3.
Literature summary: CN involvement of patients with HNCSCC and CPNI
| Series | Total number of patients | Involved nerves | V+VII involvement |
|---|---|---|---|
| Cottel et. al.24 | 14 | V1-6 V2-4 Auriculotemporal nerve-3 VII-1 Greater occipital nerve-1 III, IV and VI-1* |
2† |
| Garcia-Serra et. al.4 | 76 | VII-23+7* V1-10 V2-34+4* V3-11+10* II-1* III-4* IV-3* VI-7* |
At presentation-5% Secondarily-20%† |
| Catalano et. al.25 | 7 | VII-7 V3-3 V2-2 III, IV, VI-1 |
4 (57%) |
| McNab et. al.8††§ | 21 | VII-14 V-20 CN III, IV or VI-14 |
14 (67%) |
| Clouston et. al.10†† | 5 | V-5 VII-5 III, IV or VI-5 |
5 (100%) |
| Morris et. al.11 | 26 | VII-15 V-17 Greater auricular nerve-4 III-2 |
Not reported |
| Ballantyne et. al.14 | 26 | V-19 VII-14 Greater auricular nerve-3 III, IV, VI VIII-2* |
11 (42%) |
| Bowyer et. al.6 | 17 | V1-12 V2-4 III, IV and VI-10 VII-5 II-4 |
4 (24%) |
| Valenzuela et. al.17††§ | 8 | V1-7 V2-3 VII-5 CN III, IV or VI-4* |
4 (50%) |
| Current series | 12 | V-11 VII-11 CN III, IV and VI-3 |
10 (92%) |
Secondary involvement
Overall incidence of involvement of more than one nerve
Periorbital tumors
The authors report symptoms rather than the involved nerves
2) Figure 3 shows the extent of the CTV for an involved CN based on patterns of failure.
These volumes reflect our clinical practice, though, elective treatment to cranial nerves has become our policy only recently, following this analysis of patterns of failure.
The patterns of failure presented in Table 2 illustrate the fact that the entire CN network, from the skin to the brainstem, is one anatomical compartment without internal barriers. As a result, disease can progress in antegrade or retrograde fashion, involve peripheral nerve branches, “resurface” in subcutaneous tissue, and progress to additional nerves through communicating branches. The CTVs should account for all these possibilities by including:
The portion of the nerve proximal to the gross disease:
○ For V1-3, the course of the affected nerve to the cavernous sinus (Figure 3a, b).
○ For CN VII, the course of the nerve to the brain stem (Figure 3c, d).
A portion of the nerve distal to the gross disease.
The skin innervated by the diseased nerve, with the highest risk being at the primary tumor site.
Major communicating branches (Figure 3c, d, Figure 4b).
The compartment in which the nerve is embedded and to which it gives branches (e.g., the orbit for V1 and V2, the masticator space for V3, and the parotid gland for CN VII)
These targets would generally be defined as high risk CTVs, unless they are remote from the gross disease.
3) Optimization of RT planning
MRI has a key role both for clinical evaluation as well as for RT planning in HNCSCC with PNI. In the current series, the sensitivity of MRI was 89% (C.I. 52–99%), and the reported sensitivity and specificity of MRI in the literature are 95–100% and 95–85% respectively 18, 19. However, MRI may underestimate disease extent, accounting for reported accuracy of only 63% 18. In this series MRI showed disease progression in three asymptomatic cases. In another series MRI showed CN involvement in two of 11 asymptomatic patients20. MRI is therefore recommended to assess and define the extent of the GTV for cases with CPNI or high risk MPNI.
Organs at risk in cases of CPNI may not only be adjacent to the targets, but may actually be contained within the target (Figure 3c). Our current approach is to define the targets and prescribe doses based on the guidelines outlined below. We assign individual dose limits to the organs at risk which may supersede target dose goals such that “cold areas” are produced around organs at risk in the target. For example, the dose to the cochlea is limited to 45 Gy in cases of adjuvant RT for CN VII21 and the dose to the retina and optic nerve/chiasm is kept below 45 Gy and 60 Gy, respectively, when the cavernous sinus or orbit are part of the CTV22, 23. Higher dose limits are acceptable if these structures are involved with gross disease. In all cases, an effort should be made to reduce the dose to the contralateral vision pathways as much as possible. An expansion of 3mm is used for the critical organs at risk to account for set-up uncertainty, and daily imaging and set-up correction is practiced to ensure correct set-up.
Differential doses are prescribed to GTV, high risk CTV and low risk CTV (typically, 70Gy, 60–63 Gy, and 54–56 Gy, all in 35 fractions, at 2.0 Gy, 1.7–1.8 Gy, and 1.6 Gy per fraction, respectively). Combination of RT with chemotherapy or targeted therapies may be considered in cases with CPNI. These issues may be addressed in a similar manner as in cases of mucosal HNSCC; however, no similar data from randomized studies are available for HNCSCC.
Summary
In order to optimize local control with conformal RT or IMRT for HNCSCC with PNI, the various routes of disease spread have to be accounted for in the process of RT planning. Although rarely evident at presentation, eventual involvement of multiple CNs is common and may be the cause of local failure. It usually follows several predictable patterns depending on the primary tumor site. These patterns, described in the current paper, can serve for rational decision-making about the targets, especially the CTVs, in cases of PNI. Future studies are needed to improve risk assessment in cases with MPNI and to explore the safety and potential benefit of combining RT with chemotherapy or targeted agents.
Acknowledgments
Dr. Gluck was supported by the Trico Foundation.
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rowe DE, Carroll RJ, Day CL., Jr Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976–90. doi: 10.1016/0190-9622(92)70144-5. [DOI] [PubMed] [Google Scholar]
- 2.Mendenhall WM, Amdur RJ, Hinerman RW, et al. Skin cancer of the head and neck with perineural invasion. Am J Clin Oncol. 2007;30:93–6. doi: 10.1097/01.coc.0000251224.16075.60. [DOI] [PubMed] [Google Scholar]
- 3.Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542–7. doi: 10.1016/0002-9610(84)90385-4. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Serra A, Hinerman RW, Mendenhall WM, et al. Carcinoma of the skin with perineural invasion. Head Neck. 2003;25:1027–33. doi: 10.1002/hed.10334. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim M, Parmar H, Gandhi D, et al. Imaging nuances of perineural spread of head and neck malignancies. J Neuroophthalmol. 2007;27:129–37. doi: 10.1097/WNO.0b013e318067b8eb. [DOI] [PubMed] [Google Scholar]
- 6.Bowyer JD, Sullivan TJ, Whitehead KJ, et al. The management of perineural spread of squamous cell carcinoma to the ocular adnexae. Ophthal Plast Reconstr Surg. 2003;19:275–81. doi: 10.1097/01.IOP.0000075795.19917.B5. [DOI] [PubMed] [Google Scholar]
- 7.Galloway TJ, Morris CG, Mancuso AA, et al. Impact of radiographic findings on prognosis for skin carcinoma with clinical perineural invasion. Cancer. 2005;103:1254–7. doi: 10.1002/cncr.20913. [DOI] [PubMed] [Google Scholar]
- 8.McNab AA, Francis IC, Benger R, et al. Perineural spread of cutaneous squamous cell carcinoma via the orbit. Clinical features and outcome in 21 cases. Ophthalmology. 1997;104:1457–62. doi: 10.1016/s0161-6420(97)30116-x. [DOI] [PubMed] [Google Scholar]
- 9.McCord MW, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with incidental microscopic perineural invasion. Int J Radiat Oncol Biol Phys. 1999;43:591–5. doi: 10.1016/s0360-3016(98)00474-x. [DOI] [PubMed] [Google Scholar]
- 10.Clouston PD, Sharpe DM, Corbett AJ, et al. Perineural spread of cutaneous head and neck cancer. Its orbital and central neurologic complications. Arch Neurol. 1990;47:73–7. doi: 10.1001/archneur.1990.00530010091025. [DOI] [PubMed] [Google Scholar]
- 11.Morris JG, Joffe R. Perineural spread of cutaneous basal and squamous cell carcinomas. The clinical appearance of spread into the trigeminal and facial nerves. Arch Neurol. 1983;40:424–9. doi: 10.1001/archneur.1983.04050070054013. [DOI] [PubMed] [Google Scholar]
- 12.Schmalfuss IM, Tart RP, Mukherji S, et al. Perineural tumor spread along the auriculotemporal nerve. AJNR Am J Neuroradiol. 2002;23:303–11. [PMC free article] [PubMed] [Google Scholar]
- 13.Ginsberg LE, De Monte F, Gillenwater AM. Greater superficial petrosal nerve: anatomy and MR findings in perineural tumor spread. AJNR Am J Neuroradiol. 1996;17:389–93. [PMC free article] [PubMed] [Google Scholar]
- 14.Ballantyne AJ. Perineural invasion by SCC. J Dermatol Surg Oncol. 1984;10:502–4. doi: 10.1111/j.1524-4725.1984.tb01243.x. [DOI] [PubMed] [Google Scholar]
- 15.Soparkar CN, Patrinely JR. Squamous cell carcinoma with perineural spread. Ophthal Plast Reconstr Surg. 2001;17:470–2. doi: 10.1097/00002341-200111000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Veness MJ. Perineural spread in head and neck skin cancer. Australas J Dermatol. 2000;41:117–9. doi: 10.1046/j.1440-0960.2000.00408.x. [DOI] [PubMed] [Google Scholar]
- 17.Valenzuela AA, Whitehead KJ, Sullivan TJ. Ocular adnexal pseudo-cyst formation as a characteristic feature of perineural spread in squamous cell carcinoma. Ophthal Plast Reconstr Surg. 2006;22:201–5. doi: 10.1097/01.iop.0000218259.19584.a8. [DOI] [PubMed] [Google Scholar]
- 18.Nemzek WR, Hecht S, Gandour-Edwards R, et al. Perineural spread of head and neck tumors: how accurate is MR imaging? AJNR Am J Neuroradiol. 1998;19:701–6. [PMC free article] [PubMed] [Google Scholar]
- 19.Hanna E, Vural E, Prokopakis E, et al. The sensitivity and specificity of high-resolution imaging in evaluating perineural spread of adenoid cystic carcinoma to the skull base. Arch Otolaryngol Head Neck Surg. 2007;133:541–5. doi: 10.1001/archotol.133.6.541. [DOI] [PubMed] [Google Scholar]
- 20.Williams LS, Mancuso AA, Mendenhall WM. Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int J Radiat Oncol Biol Phys. 2001;49:1061–9. doi: 10.1016/s0360-3016(00)01407-3. [DOI] [PubMed] [Google Scholar]
- 21.Pan CC, Eisbruch A, Lee JS, et al. Prospective study of inner ear radiation dose and hearing loss in head-and-neck cancer patients. Int J Radiat Oncol Biol Phys. 2005;61:1393–402. doi: 10.1016/j.ijrobp.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Parsons JT, Bova FJ, Fitzgerald CR, et al. Radiation optic neuropathy after megavoltage external-beam irradiation: analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30:755–63. doi: 10.1016/0360-3016(94)90346-8. [DOI] [PubMed] [Google Scholar]
- 23.Monroe AT, Bhandare N, Morris CG, et al. Preventing radiation retinopathy with hyperfractionation. Int J Radiat Oncol Biol Phys. 2005;61:856–64. doi: 10.1016/j.ijrobp.2004.07.664. [DOI] [PubMed] [Google Scholar]
- 24.Cottel WI. Perineural invasion by squamous-cell carcinoma. J Dermatol Surg Oncol. 1982;8:589–600. doi: 10.1111/j.1524-4725.1982.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 25.Catalano PJ, Sen C, Biller HF. Cranial neuropathy secondary to perineural spread of cutaneous malignancies. Am J Otol. 1995;16:772–7. [PubMed] [Google Scholar]