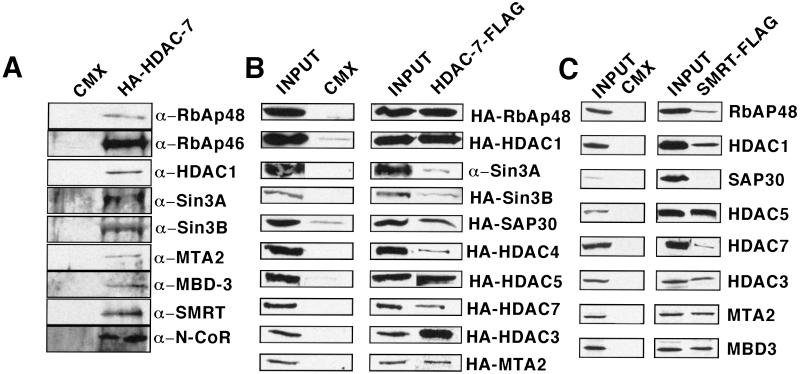
Figure 4.
Members of the Sin3/HDAC and NuRD/Mi2 deacetylase protein complexes can interact with Class II HDAC7 and SMRTa. (A) HDAC7 complexes in 293 cells with proteins that have been characterized in the Sin3/HDAC and NuRD/Mi2 complexes. Whole-cell extracts prepared from 293 cells with either vector alone or mHDAC7-HA expression vector were incubated with anti-HA antibodies conjugated with agarose beads. Immunoprecipitates were subjected to Western blot analysis and probed with antibodies to RbAp48, RbAp46 (Upstate Biotechnology), HDAC1 (Affinity BioReagents), mSin3A/B (Santa Cruz), MTA2 (D. Reinberg, Howard Hughes Medical Institute, Piscataway, NJ), MBD3 (D. Reinberg), SMRT (Affinity BioReagents), and N-CoR (Upstate Biotechnology). (B) Results of coimmunoprecipitation experiments demonstrating interaction of HDAC7 with members of the Sin3/HDAC and NuRD/Mi2 deacetylase protein complexes. 293 cells were transfected with empty vector or Flag-tagged HDAC7 with the vector containing the DNA of interest HA-tagged. Whole-cell extracts were immunoprecipitated with FLAG antibody subjected to Western blot analysis and probed with anti-HA antibody. Inputs to the corresponding lanes (Left). (C) Results of coimmunoprecipitation experiments by using Flag-tagged SMRTa and HA-tagged members of the Sin3/HDAC and NuRD/Mi2 deacetylase protein complexes in 293 cells. Coimmunoprecipitation experiments were done as previously described