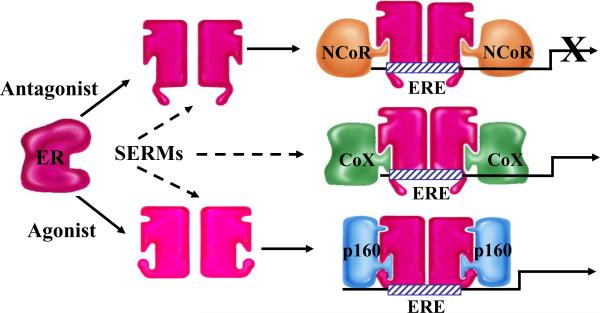
Box 1. Cofactor Proteins and SERM Action.
Upon binding ligand, the estrogen receptor (ER) undergoes a conformational change that permits spontaneous homo- or hetero-dimerization and facilitates its subsequent interaction with estrogen response elements (EREs) located within target genes or with other transcription factors and transcription factor complexes. The functional consequences of different ligand-induced conformational changes were revealed with the discovery of receptor coactivators and corepressors.
Coactivators are proteins that interact either directly or indirectly with activated nuclear receptors (NRs) and facilitate their transcriptional activation. Coactivators often posses enzymatic activity, such as acetyltransferase, methyltransferase, or ubiquitin ligase activity. These proteins also may function as a bridge between an NR and the general transcription machinery. Examples include the p160 family members SRC-1, SRC-2 (GRIP1), and SRC-3 (ACTR, AIB1).
Corepressors also interact either directly or indirectly with NR family members. In contrast to coactivators, corepressors can bind to NRs in the absence or presence of a ligand, depending on the character of that particular ligand, and maintain the receptor in a quiescent state. Corepressors are often part of large protein complexes that contain histone deacetylase activity, and thus repress gene transcription. Examples include NCoR and SMRT.
In general, Selective Estrogen Receptor Modulators (SERMs) permit the receptor to adopt a structure that is intermediate between the inactive and fully activated states. Thus, the relative agonist/antagonist activity of SERMs is a reflection of the ability of the ER-SERM complex to engage coactivators or corepressors. It is also possible that some SERMs allow ER to interact in an ectopic manner with coregulatory proteins (CoX) that would not interact with ER under normal physiological circumstances.