Abstract
Background
Brain-derived neurotrophic factor (BDNF) mutant mice show hyperphagia and hyperleptinemia. Animal and cell-culture experiments suggest multiple interrelations between BDNF and the serotonin (5-HT) system. We studied serum BDNF in patients with anorexia nervosa and its associations with peripheral indicators of the 5-HT system. To control for secondary effects of acute malnutrition, we assessed acutely underweight patients with anorexia nervosa (acAN) in comparison to long-term weight-recovered patients with the disorder (recAN) and healthy controls.
Methods
We determined serum BDNF, platelet 5-HT content and platelet 5-HT uptake in 33 patients in the acAN group, 20 patients in the recAN group and 33 controls. Plasma leptin served as an indicator of malnutrition.
Results
Patients in the acAN group were aged 14–29 years and had a mean body mass index (BMI) of 14.9 (standard deviation [SD] 1.4) kg/m2. Those in the recAN group were aged 15–29 years and had a mean BMI of 20.5 (SD 1.3) kg/m2 and the controls were aged 15–26 years and had a BMI of 21.4 (SD 2.1) kg/m2. The mean serum BDNF levels were significantly increased in the recAN group compared with the acAN group (8820, SD 3074 v. 6161, SD 2885 pg/mL, U = 154.5, p = 0.001). There were no significant associations between BDNF and either platelet 5-HT content or platelet 5-HT uptake. Among patients with anorexia nervosa, we found significant positive linear relations between BDNF and BMI (r = 0.312, p = 0.023) and between BDNF and leptin (r = 0.365, p = 0.016).
Limitations
We measured the signal proteins under study in peripheral blood.
Conclusion
Serum BDNF levels in patients with anorexia nervosa depend on the state of illness and the degree of hypoleptinemia. Upregulation of BDNF in weight-recovered patients with anorexia nervosa could be part of a regenerative process after biochemical and molecular neuronal injury due to prolonged malnutrition. Associations between the BDNF and the 5-HT system in humans remain to be established.
Introduction
Anorexia nervosa is a frequent disorder among adolescent girls and young women. According to the DSM-IV, diagnostic criteria for anorexia nervosa consist of weight loss or failure to attain expected weight gain during periods of growth, intense fear of gaining weight or becoming fat, body image disturbance and amenorrhea. Arising during the period of growth and maturation, anorexia nervosa leads to interruptions of somatic and psychological development and to serious medical complications and high mortality.
It is well known from computed tomography and magnetic resonance imaging studies that underweight patients with anorexia nervosa have enlarged sulci and ventricles, cortical atrophy and decreased grey and white matter volumes.1 Changes in brain metabolism as measured by magnetic resonance spectroscopy seem to be related to poor cognitive function.2
Brain-derived neurotrophic factor (BDNF) is widely and abundantly expressed in the central nervous system and is available to some peripheral neurons.3,4 It has been implicated in the development of the nervous system (i.e., neuronal growth, differentiation, synaptic connectivity) and in neuronal survival and repair.5,6 Changes in central or peripheral BDNF concentrations have been linked to a wide variety of conditions. In particular, a decreased production of BDNF protein has been found in neurodegenerative disorders7 and in affective disorders.8
Several lines of evidence suggest that BDNF plays a role in the pathophysiology of eating disorders. First, BDNF conditional mutant mice and BDNF+/− mutants show hyperphagia, hyperleptinemia and an age-related increase in body weight.9–12 Second, exogenous BDNF treatment has been demonstrated to transiently reverse these abnormal eating behaviours and obesity in BDNF+/− mice.10 Third, in mice, fasting reduces BDNF, and leptin is able to induce the expression of BDNF in the hypothalamus via the melanocortin pathway.13,14 Fourth, associations between BDNF gene polymorphisms and anorexia nervosa have been demonstrated.15–17 Studies on peripheral BDNF levels in humans with eating disorders have been somewhat contradictory, but most authors reported decreased serum BDNF levels in patients with anorexia nervosa or bulimia nervosa.18–21 However, one large investigation demonstrated lower serum BDNF in extremely overweight children and adolescents.22 It remains to be elucidated whether decreased BDNF levels are a mere consequence of prolonged cachexia or a stable trait marker in patients with anorexia nervosa. To date, only one small study has attempted to determine BDNF levels in patients with anorexia nervosa after partial weight recovery.18
Another important signalling system in anorexia nervosa is the serotonin (5-HT) system. Pharmacological agents that increase intrasynaptic 5-HT or directly activate 5-HT receptors tend to reduce food consumption, whereas interventions lowering 5-HT neurotransmission increase food consumption and promote weight gain.23 A number of studies have shown reduced 5-HT activity in acutely underweight patients with anorexia nervosa.24–26 In contrast, for patients with anorexia nervosa after long-term weight recovery, increased 5-HT transmission has been suggested as a trait marker of the disorder.27,28
Brain-derived neurotrophic factor and 5-HT are 2 seemingly distinct signalling systems; however, BDNF has been repeatedly shown to promote the survival and differentiation of 5-HT neurons in vitro. Conversely, administration of anti-depressants enhances BDNF gene expression.29
We aimed to answer 2 major research questions. First, we tried to eliminate secondary effects of acute malnutrition on BDNF by studying long-term weight-restored patients with anorexia nervosa in comparison with acutely underweight patients with anorexia nervosa and healthy controls. Therefore, we also determined circulating leptin concentrations as a biological marker for the degree of malnutrition. Leptin is a nutritionally regulated hormone primarily secreted by adipocytes that affects food intake and energy expenditure.30 Hypoleptinemia is indicative of progressed semistarvation, an exceedingly low fat mass and impaired function of the hypothalamic–pituitary–gonadal axis.31 Second, we hypothesized an association between serum BDNF and peripheral markers of the 5-HT system. We assessed platelet 5-HT uptake with low substrate concentrations and platelet 5-HT content. The human platelet 5-HT transporter and the brain 5-HT transporter are identical proteins that are encoded by the same single-copy gene.32 Recent ex-vivo and neuroimaging data provide evidence for functional similarities between the neuronal and platelet 5-HT transporter.33,34
Methods
Participants
The sample population consisted of female patients with acute anorexia nervosa, short-term/partially weight-restored anorexia nervosa, recovered anorexia nervosa and healthy controls. We recruited participants with acute anorexia nervosa (acAN group) according to DSM-IV criteria within 1 week after admittance to eating disorder programs of a university child and adolescent psychiatry and psychosomatic medicine department. During the study period, all acAN patients were enrolled in a behaviourally oriented nutritional rehabilitation program and were encouraged to gain a minimum of 500 g of body weight weekly. Following a weight gain of 10% or more, we reassessed the patients; we refer to them as patients with “short-term/partially weight-restored anorexia nervosa.”
We also included 20 participants who had been previously treated for anorexia nervosa and who had successfully recovered from their illness (recAN group). To be considered “recovered,” participants had to
maintain a body mass index (BMI) greater than 18.5 kg/m2 (patients older than 18 yr) or less than the tenth BMI percentile (patients younger than 18 yr35) for at least 3 months before the study;
menstruate; and
refrain from binging, purging or engaging in substantially restrictive eating patterns.
The control group consisted of normal-weight, eumenorrhoeic, healthy participants who we recruited through advertisement among middle school, high school and university students.
We obtained information on possible confounding variables, including menstrual cycle and use of contraceptive medication, using a semistructured research interview (the Structured Interview of Anorexia Nervosa and Bulimic Syndromes [SIAB-EX]36 interview) and by physical examination. We derived information on comorbid psychiatric diagnoses other than eating disorders from medical records. We also assessed smoking habits, but they have not been reported to affect 5-HT uptake or concentration in platelets.
We excluded controls if they had any history of psychiatric illness. We excluded patients if they had a lifetime history of any of the following clinical diagnoses: organic brain syndrome, schizophrenia, substance dependence, bipolar disorder, bulimia nervosa or binge-eating disorder. Further exclusion criteria for all participants were IQ lower than 85; current inflammatory, neurologic or metabolic illness; chronic bowel diseases; cancer; anemia; pregnancy; breast feeding; current use of acetylsalicylic acid, cortisone, antibiotics or antihypertensive medication; and use of anti-depressants, antipsychotics or any other psychotropic medications or substances within the 6 weeks preceding the study.
The Institutional Review Board of the Charité —Universitätsmedizin Berlin approved our study. All participants, or their guardians if the participants were underage, provided written informed consent.
Clinical measures
We assessed current and/or past eating disorders in all participants using the expert form of the SIAB-EX.36 The SIAB-EX for participants aged 12–65 years is a semistandardized interview that assesses the prevalence and severity of specific eating-related psychopathology over a period of 3 months according to DSM-IV diagnostic criteria. It consists of 87 items and provides diagnoses of eating disorders according to International Classification of Diseases, version 10 and DSM-IV criteria. Internal consistency was good, and the inter-rater reliability ranged from 0.86 to 0.96.37 Clinically experienced and trained research assistants (S.E., J.V.M.) conducted the interviews under the supervision of an attending child and adolescent psychiatrist (E.P., U.L.).
We assessed eating disorder–specific psychopathology using the short version of the Eating Disorders Inventory (EDI-2), a self-report questionnaire comprising 8 subscales.38 Response categories range from 1 “never” to 6 “always.” The 3 core subscales “drive for thinness,” “body dissatisfaction” and “bulimia” were part of the confirmatory analyses in the present study.
We determined general levels of psychopathology using the global severity index of the Symptom Checklist-90-Revised (SCL-90-R).39
Blood collection and biochemical assessments
To avoid possible confounding related to the season of testing, we recruited and assessed patients and controls in parallel. We collected venous blood samples of 9 mL into vacutainer tubes between 7:30 and 9:30 am after overnight fasting. We centrifuged serum samples (800 g for 15 min) and stored them at − 80°C.
We measured endogenous levels of BDNF in the thawed serum samples using commercial enzyme-linked immunosorbent assay (ELISA; Promega Inc.) kits in principle according to the manufacturer’s instructions but adapted to the fluorometric technique used also for nerve growth factor determination and described in detail previously.40,41 We expressed the BDNF content as equivalents of recombinant human BDNF. The detection limit of the assay was 1 pg/mL. Determinations of recovery, specific and unspecific neurotrophin binding (the latter against mouse IgG1 κ monoclonal isotype control) involved quadruplicate fluorescence determinations for each serum sample.
We prepared platelet-rich plasma and performed assays of 5-HT uptake and concentration immediately after venipuncture, as described elsewhere.42 In brief, we measured platelet 5HT content by high-performance liquid chromatography (HPLC) with electrochemical detection after deproteinization of the samples with perchloric acid. We examined 5-HT uptake by incubation of 100 μL platelet-rich plasma (3.5–4.5 × 107 platelets) in Krebs phosphate buffer (pH 7.4) at very low substrate concentration (15 nM [14C]-5-HT) at 37°C for 5 minutes (final volume 500 μL). After incubation, we collected and washed platelets by filtration on glass microfibre filters (Whatman).
We measured plasma leptin concentrations using a commercial Human Leptin “Dual Range” ELISA kit (Millipore) according to the manufacturer’s instructions. Depending on the protocol, sensitivity was 0.125–20 ng/mL or 0.5–100 ng/mL.
Statistical analysis
Unless indicated otherwise, we presented all values as means and standard deviations (SD). We used histograms, box plots, normal probability plots and Levene statistics to verify the underlying statistical assumptions. Owing to violations of homogeneity of variances for plasma leptin concentrations and non-normality for BDNF levels, we performed comparisons including these indicators with nonparametric methods such as Kruskal–Wallis 1-way analysis of variance (ANOVA), Mann–Whitney U tests and Wilcoxon tests. We compared all other variables using 1-way ANOVA with subsequent Scheffé post-hoc tests and paired t tests.
Because deviations from normality for BDNF were marginal in this study, we also used analysis of covariance (ANCOVA) to control for the effects of possible confounding variables. Age served as a covariate. We entered use of contraceptive medication and menstrual cycle as random cofactors. The variable used for menstrual cycle had 3 levels according to the presumed hormonal situation: follicular phase (high estradiol), luteal phase (high estradiol and progesterone) and amenorrhoea or irregular cycle (> 45 d, low estradiol and progesterone).
We calculated correlations using Spearman correlation coefficients. We analyzed measures of psychopathology as a group by multivariate analyses of variance (MANOVA). We subsequently performed a Tamhane post-hoc test for multiple comparisons as the homogeneity of variance assumption was not met. We performed all tests with SPSS statistical software, version 14.0 (SPSS Inc.).
Results
Participants
We enrolled 33 participants aged 14–29 years in the acAN group. Of these, 21 (64%) of the patients were of the restrictive and 12 (36%) were of the binge/purge subtype, and 8 (24%) had comorbid psychiatric disorders (5 had depressive disorders, including dysthymia, and 3 had obsessive–compulsive disorder). Following a weight gain of 10% or more, we reassessed 7 (21%) patients, who we refer to as patients with short-term/partially weight-restored anorexia nervosa. We enrolled 20 participants aged 15–29 years in the recAN group. Of these, 6 (30%) had associated psychiatric comorbidity at the time of treatment (5 had depressive disorders, including dysthymia, and 1 had other disorders). Finally, we enrolled 33 normal-weight, eumenorrhoeic, healthy participants aged 15–26 years in the control group.
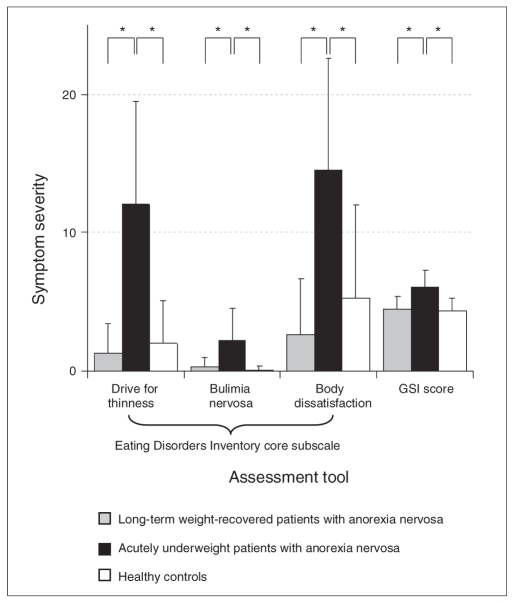
Figure 1 shows the mean values of the EDI-2 core subscales and the SCL-90-R summary scale for participants in the acAN, recAN and control groups. Results of the MANOVA indicated significant differences among the 3 groups for all EDI-2 subscales and the SCL-90-R summary scale (Pillai criterion F8 = 8.2, p < 0.001). Tamhane post-hoc tests revealed that patients in the acAN group scored significantly higher than those in the recAN and control groups on all aforementioned scales. We observed no statistically significant differences between the recAN and control groups.
Fig. 1.
Mean scores and standard deviations on the Eating Disorders Inventory-238 core subscales and the Symptom Checklist-90-Revised39 Global Severity Index (T score × 10−1) for long-term weight-recovered patients with anorexia nervosa (recAN group), acutely underweight patients with anorexia nervosa (acAN group) and controls. Significant differences on Tamhane post-hoc tests for multiple comparisons between the acAN and control groups and between the acAN and recAN groups are indicated by asterisks (p < 0.001). There were no statistically significant differences between the recAN and control groups (p = 0.65 for drive for thinness, p = 0.45 for bulimia nervosa, p = 0.23 for body dissatisfaction and p = 0.98 for Global Severity Index).
General characteristics and 5-HT platelet variables of the 3 groups are summarized in Table 1. Age, 5-HT uptake and 5-HT content were similar among the 3 groups, but BMI and plasma leptin concentrations were significantly lower in the acAN group than in the recAN and control groups.
Table 1.
Comparison of demographic and clinical characteristics of 53 adolescent girls and women with anorexia nervosa and 33 matched controls
| Group; mean (SD)
|
|||||
|---|---|---|---|---|---|
| Characteristic | acAN (n = 33) | recAN (n = 20) | Control (n = 33) | Statistical test* | p value |
| Age, yr | 18.9 (3.9)† | 21.0(3.9) † | 19.0 (3.1) | F2,83 = 2.48 | 0.09 |
| BMI, kg/m2 | 14.9 (1.4) †‡ | 20.5(1.3) † | 21.4 (2.1)‡ | F2,83 = 138.31 | < 0.001 |
| Leptin, ng/Ml | 1.1 (1.7) †‡ | 9.2(6.5)‡ | 15.3 (8.7)‡ | χ22 = 83.69 | < 0.001 |
| 5-HT content, ng 5-HT/109 platelets | 520.4 (206.8) | 411.8(160.1) | 452.8 (146.0) | F2,83 = 2.52 | 0.09 |
| 5-HT uptake, pMol 14C-5-HT/109 platelets × 5 min | 62.5 (20.1) | 61.4(11.1) | 56.8 (11.3) | F2,83 = 1.21 | 0.30 |
5-HT = serotonin; acAN = acutely underweight patients with anorexia nervosa; BMI = body mass index; recAN = long-term weight-recovered patients with anorexia nervosa; SD = standard deviation.
Cross-sectional analyses were conducted by analysis of variance, except for leptin, which was calculated using Kruskal–Wallis 1-way analysis of variance.
Values differ on Mann–Whitney U tests at the p < 0.001 level.
Values differ on Scheffé post-hoc tests for multiple comparisons at the p < 0.05 level or less.
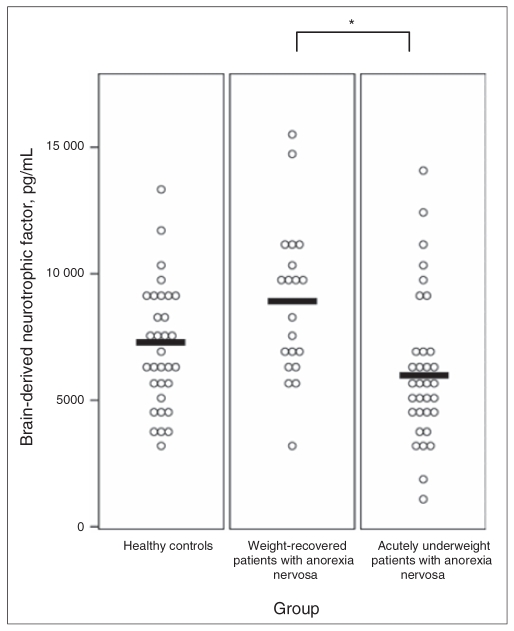
Regarding our main outcome parameter, serum BDNF (Fig. 2), results of the Kruskal–Wallis 1-way ANOVA indicated significant overall group differences (χ2 = 11.05, p = 0.004). Pair-wise Mann–Whitney U tests revealed significantly higher serum BDNF in the recAN group than in the acAN group (U = 154.5, p = 0.001). In addition, we found that BDNF levels were lower in the control group than in the recAN group (U = 226.0, p = 0.06) and lower in acAN group than in the control group (U = 408.0, p = 0.08). To address possible confounding related to age, use of contraceptive medications and phase of menstrual cycle, we reanalysed our data with ANCOVA. Neither age (F1,85 = 2.53, p = 0.12) nor use of contraceptive medications (F1,85 = 1.55, p = 0.22) and menstrual cycle (F2,83 = 0.50, p = 0.74) showed a significant effect in the ANCOVA models. An exploratory subanalysis comparing patients in the acAN group of the restrictive subtype with those of the binge/purge subtype revealed no statistically significant differences between the 2 subgroups (5406.5, SD 1959.7 v. 7481.8, SD 1089.4 pg/mL; U = 93.0, p = 0.23), but the mean serum BDNF level of patients of the restrictive subtype (n = 21) was significantly lower than that of patients in the control group (U = 215.5, p = 0.020).
Fig. 2.
Individual values and group means of serum brain-derived neurotrophic factor in healthy controls, long-term weight-recovered patients with anorexia nervosa and acutely underweight patients with anorexia nervosa. The significant statistical difference between the anorexia groups is indicated by an asterisk (p < 0.005).
We performed exploratory correlational analyses between cross-sectionally determined serum BDNF and nutritional variables (BMI, leptin), psychological measures (EDI-2 core subscales, SCL-90-R global severity index) and 5-HT parameters (5-HT uptake, 5-HT content) for patients with anorexia nervosa (recAN + acAN) and controls separately. Among patients, we found significant positive linear relations between BDNF and BMI (r = 0.312, p = 0.023) and between BDNF and leptin (r = 0.365, p = 0.016). We found significant negative linear relations between BDNF and “drive for thinness” (r = − 0.312, p = 0.026), BDNF and “body dissatisfaction” (r = − 0.450, p = 0.001) and BDNF and the Global Severity Index (r = − 0.283, p = 0.044). When considered separately, we observed no significant linear relations in the 3 groups.
We reinvestigated 7 patients in the acAN group at a second time point (T2, mean 12.8, SD 4.0 weeks after the first assessment) after a short-term/partial weight recovery (weight gain ≥ 10%). We observed a statistical trend for higher plasma leptin at the second assessment compared with the first (mean 0.4, SD 0.4 v. 5.2, SD 6.4 ng/mL; Z = − 1.83, p = 0.07), but there was no significant change in serum BDNF (mean 6168.3, SD 2558.0 v. 7337.9, SD 1836.7 pg/mL; Z = − 1.35, p = 0.18).
Discussion
To our knowledge, this is the first study investigating BDNF serum levels in recovered patients with anorexia nervosa in comparison to acutely underweight patients with the disorder and healthy controls. We found significantly elevated BDNF serum concentrations in recovered patients with anorexia nervosa compared with patients in the acute stage of illness. Our results underline the significance of malnutrition and hypoleptinemia for the interpretation of changes in peripheral BDNF concentrations in patients with this disorder.
Given that cerebral BDNF crosses the blood–brain barrier,43 it is reasonable to assume that serum BDNF concentrations are associated with BDNF levels in the brain. This assumption is substantiated by animal experiments showing that BDNF in serum is correlated with BDNF expression in cortical brain regions44 and the recently demonstrated positive association between N-acetylaspartate, a well established marker of neuronal integrity, and serum BDNF in healthy humans.45,46 Hence, upregulation of BDNF could be part of a regenerative process associated with weight recovery.4 The significant correlations that we observed between serum BDNF and leptin or BMI in the whole group of patients with anorexia nervosa but not in controls lends support to this hypothesis. Previous studies in patients with eating disorders also found a positive relation between BMI and serum BDNF,18–21 whereas no associations or inverse relations exist in normal healthy volunteers.41,47,48 Since there was only a statistical trend for the difference in serum BDNF between recovered patients with anorexia nervosa and healthy controls, BDNF in recovered patients is either normalized or truly elevated. However, it remains speculative whether higher BDNF concentrations can reverse the detrimental effects of prolonged malnutrition on neural networks in patients with anorexia nervosa.
Furthermore, animal experiments provide evidence for a role of BDNF in the regulation of food intake and energy homeostasis. Accordingly, we and others found, in particular, eating disorder–specific symptoms to be associated with lower BDNF levels.18,49 In line with that, most previous studies demonstrated decreased BDNF serum levels in patients with acute anorexia nervosa.18–21 In our sample, we found a statistical trend for lower serum BDNF in all patients in the acAN group and significantly decreased BDNF in patients of the restrictive subtype. In contrast, Mercader and colleagues15 reported elevated plasma BDNF in a mixed sample of patients with anorexia nervosa and bulemia nervosa. Differences between studies might be attributed to the methodology used to determine serum BDNF, the assessment of BDNF in plasma or whole blood instead of serum, or to sample characteristics (e.g., different proportions of anorexia nervosa subtypes within the patient group or unaffected discordant siblings as the comparison group in the study by Mercader and colleagues). To date only one other investigation aimed to measure BDNF longitudinally. Similar to our results, there was no significant difference in serum BDNF in the acute stage and after partial weight recovery.18 Full weight recovery might be a prerequisite for an increase of serum BDNF.
A multitude of animal studies and cell-culture experiments suggest that BDNF promotes the development and function of 5-HT neurons. In particular, BDNF promotes the sprouting of embryonic, mature and injured 5-HT axons.50–52 Further studies suggest that 5-HT transmission exerts powerful control over BDNF expression.29 There is only limited ex-vivo evidence for the interrelations of the neurotrophin and the 5-HT system in humans. In the present study, we failed to detect significant associations between BDNF and either platelet 5-HT content or platelet 5-HT uptake. Possible explanations are differences in the experimental design and between species. Cell-culture and animal experiments dealing with the relation between BDNF and the 5-HT system are mostly based on supraphysiological BDNF concentrations and are therefore not easily transferred to the in-vivo situation.12,51 In addition, there might be differences in the regulation of platelet and neuronal 5-HT uptake. However, an association between low BDNF levels and low levels of central 5-HT activity using evidence from auditory signal processing has been demonstrated previously.53 Other studies have found conflicting evidence regarding the genetic epistasis between the 5-HT transporter-linked polymorphic region and the BDNF val66met polymorphism in humans.54,55
Limitations
The findings of our study should be considered in light of the following limitations. First, the sample size in the longitudinal arm of the study was too small to draw reliable conclusions. In addition, partial weight recovery might not have been sufficient to correctly assess regenerative processes. Second, the signal proteins investigated in our study were measured in peripheral blood. However, there is considerable evidence that they reflect the actual condition in the central nervous system.
In summary, results of our study suggest an effect of malnutrition and hypoleptinemia on peripheral BDNF concentrations in patients with anorexia nervosa. Recovered patients have elevated BDNF levels compared with acutely underweight patients. This might be part of a regenerative process after biochemical and molecular neuronal injury. It may also be related to the regulation of appetite and eating behaviour. There were no associations between BDNF and peripheral markers of 5-HT transmission. Large prospective studies are needed to verify our findings and clarify the effect of BDNF on appetite before the onset and after recovery from anorexia nervosa within the same patients.
Acknowledgements
The authors would like to thank Mrs. I. Kamenzky for excellent technical assistance and Mrs. D. Weiss for help with the biochemical assessments.
Footnotes
Competing interests: None declared for Ms. Eckart and Merle and Drs. Ehrlich, Salbach-Andrae, Burghardt, Pfeiffer, Franke, Uelbelhack and Lehmkuhl. Dr. Hellweg has travel assistance from Merz Pharmaceutical and Eli Lilly and speaker fees from Merz Pharmaceutical, Pfizer, GlaxoSmithKline, Janssen and Novartis.
Contributors: Drs. Ehrlich, Salbach-Andrae, Pfeiffer, Lehmkuhl and Hellweg designed the study. Ms. Eckart and Merle and Drs. Ehrlich, Burghardt, Franke and Hellweg acquired the data, which Drs. Ehrlich, Salbach-Andrae, Franke, Uebelhack and Hellweg analyzed. Dr. Ehrlich wrote the article, which all other authors reviewed. All authors approved the final version for publication.
References
- 1.Kerem NC, Katzman DK. Brain structure and function in adolescents with anorexia nervosa. Adolesc Med. 2003;14:109–18. [PubMed] [Google Scholar]
- 2.Ohrmann P, Kersting A, Suslow T, et al. Proton magnetic resonance spectroscopy in anorexia nervosa: correlations with cognition. Neuroreport. 2004;15:549–53. doi: 10.1097/00001756-200403010-00033. [DOI] [PubMed] [Google Scholar]
- 3.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 4.Nockher WA, Renz H. Neurotrophins in clinical diagnostics: pathophysiology and laboratory investigation. Clin Chim Acta. 2005;352:49–74. doi: 10.1016/j.cccn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 6.Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991;14:165–70. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- 7.Schulte-Herbruggen O, Braun A, Rochlitzer S, et al. Neurotrophic factors — A tool for therapeutic strategies in neurological, neuropsychiatric and neuroimmunological diseases? Curr Med Chem. 2007;14:2318–29. doi: 10.2174/092986707781745578. [DOI] [PubMed] [Google Scholar]
- 8.Post RM. Role of BDNF in bipolar and unipolar disorder: clinical and theoretical implications. J Psychiatr Res. 2007;41:979–90. doi: 10.1016/j.jpsychires.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Lyons WE, Mamounas LA, Ricaurte GA, et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–44. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rios M, Fan G, Fekete C, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–57. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 12.Chourbaji S, Hellweg R, Brandis D, et al. Mice with reduced brain-derived neurotrophic factor expression show decreased choline acetyltransferase activity, but regular brain monoamine levels and unaltered emotional behavior. Brain Res Mol Brain Res. 2004;121:28–36. doi: 10.1016/j.molbrainres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Komori T, Morikawa Y, Nanjo K, et al. Induction of brain-derived neurotrophic factor by leptin in the ventromedial hypothalamus. Neuroscience. 2006;139:1107–15. doi: 10.1016/j.neuroscience.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 14.Xu B, Goulding EH, Zang K, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–42. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercader JM, Ribases M, Gratacos M, et al. Altered brain-derived neurotrophic factor blood levels and gene variability are associated with anorexia and bulimia. Genes Brain Behav. 2007;6:706–16. doi: 10.1111/j.1601-183X.2007.00301.x. [DOI] [PubMed] [Google Scholar]
- 16.Ribases M, Gratacos M, Armengol L, et al. Met66 in the brain-derived neurotrophic factor (BDNF) precursor is associated with anorexia nervosa restrictive type. Mol Psychiatry. 2003;8:745–51. doi: 10.1038/sj.mp.4001281. [DOI] [PubMed] [Google Scholar]
- 17.Ribases M, Gratacos M, Fernandez-Aranda F, et al. Association of BDNF with restricting anorexia nervosa and minimum body mass index: a family-based association study of eight European populations. Eur J Hum Genet. 2005;13:428–34. doi: 10.1038/sj.ejhg.5201351. [DOI] [PubMed] [Google Scholar]
- 18.Nakazato M, Hashimoto K, Yoshimura K, et al. No change between the serum brain-derived neurotrophic factor in female patients with anorexia nervosa before and after partial weight recovery. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1117–21. doi: 10.1016/j.pnpbp.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Monteleone P, Fabrazzo M, Martiadis V, et al. Circulating brain-derived neurotrophic factor is decreased in women with anorexia and bulimia nervosa but not in women with binge-eating disorder: relationships to co-morbid depression, psychopathology and hormonal variables. Psychol Med. 2005;35:897–905. doi: 10.1017/s0033291704003368. [DOI] [PubMed] [Google Scholar]
- 20.Monteleone P, Tortorella A, Martiadis V, et al. Opposite changes in the serum brain-derived neurotrophic factor in anorexia nervosa and obesity. Psychosom Med. 2004;66:744–8. doi: 10.1097/01.psy.0000138119.12956.99. [DOI] [PubMed] [Google Scholar]
- 21.Nakazato M, Hashimoto K, Shimizu E, et al. Decreased levels of serum brain-derived neurotrophic factor in female patients with eating disorders. Biol Psychiatry. 2003;54:485–90. doi: 10.1016/s0006-3223(02)01746-8. [DOI] [PubMed] [Google Scholar]
- 22.El-Gharbawy AH, Adler-Wailes DC, Mirch MC, et al. Serum brain-derived neurotrophic factor concentrations in lean and overweight children and adolescents. J Clin Endocrinol Metab. 2006;91:3548–52. doi: 10.1210/jc.2006-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blundell JE, Lawton CL, Halford JC. Serotonin, eating behavior, and fat intake. Obes Res. 1995;3(Suppl 4):471S–6S. doi: 10.1002/j.1550-8528.1995.tb00214.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaye WH, Ebert MH, Raleigh M, et al. Abnormalities in CNS monoamine metabolism in anorexia nervosa. Arch Gen Psychiatry. 1984;41:350–5. doi: 10.1001/archpsyc.1984.01790150040007. [DOI] [PubMed] [Google Scholar]
- 25.Brewerton TD, Jimerson DC. Studies of serotonin function in anorexia nervosa. Psychiatry Res. 1996;62:31–42. doi: 10.1016/0165-1781(96)02987-3. [DOI] [PubMed] [Google Scholar]
- 26.Monteleone P, Brambilla F, Bortolotti F, et al. Prolactin response to d-fenfluramine is blunted in people with anorexia nervosa. Br J Psychiatry. 1998;172:439–42. doi: 10.1192/bjp.172.5.439. [DOI] [PubMed] [Google Scholar]
- 27.Kaye WH, Frank GK, Bailer UF, et al. Serotonin alterations in anorexia and bulimia nervosa: new insights from imaging studies. Physiol Behav. 2005;85:73–81. doi: 10.1016/j.physbeh.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Ehrlich S, Franke L, Schott R, et al. Platelet monoamine oxidase activity in underweight and weight-recovered females with anorexia nervosa. Pharmacopsychiatry. 2008;41:226–31. doi: 10.1055/s-2008-1078749. [DOI] [PubMed] [Google Scholar]
- 29.Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- 30.Zhang F, Chen Y, Heiman M, et al. Leptin: structure, function and biology. Vitam Horm. 2005;71:345–72. doi: 10.1016/S0083-6729(05)71012-8. [DOI] [PubMed] [Google Scholar]
- 31.Hebebrand J, Muller TD, Holtkamp K, et al. The role of leptin in anorexia nervosa: clinical implications. Mol Psychiatry. 2007;12:23–35. doi: 10.1038/sj.mp.4001909. [DOI] [PubMed] [Google Scholar]
- 32.Lesch KP, Wolozin BL, Murphy DL, et al. Primary structure of the human platelet serotonin uptake site: identity with the brain serotonin transporter. J Neurochem. 1993;60:2319–22. doi: 10.1111/j.1471-4159.1993.tb03522.x. [DOI] [PubMed] [Google Scholar]
- 33.Rausch JL, Johnson ME, Li J, et al. Serotonin transport kinetics correlated between human platelets and brain synaptosomes. Psychopharmacology (Berl) 2005;180:391–8. doi: 10.1007/s00213-005-2178-6. [DOI] [PubMed] [Google Scholar]
- 34.Uebelhack R, Franke L, Herold N, et al. Brain and platelet serotonin transporter in humans-correlation between [123I]-ADAM SPECT and serotonergic measurements in platelets. Neurosci Lett. 2006;406:153–8. doi: 10.1016/j.neulet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Kromeyer-Hauschild K, Wabitsch M, Kunze D, et al. Perzentile für den Body Mass Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschr Kinderheilkd. 2001;149:807–18. [Google Scholar]
- 36.Fichter M, Quadflieg N. Das Strukturierte Inventar für Anorektische und Bulimische Ess-Störungen nach DSM-IV und ICD-10. Bern (Switzerland): Huber; 1999. [Google Scholar]
- 37.Fichter M, Quadflieg N. The structured interview for anorexic and bulimic disorders for DSM-IV and ICD-10 (SIAB-EX): reliability and validity. Eur Psychiatry. 2001;16:38–48. doi: 10.1016/s0924-9338(00)00534-4. [DOI] [PubMed] [Google Scholar]
- 38.Rathner G, Waldherr K. Eating Disorder Inventory-2. Eine deutschsprachige Validierung mit Normen für weibliche und männliche Jugendliche. Z Klin Psychol Psychiatr Psychother. 1997;45:157–82. [Google Scholar]
- 39.Franke GH. Die Symptom-Checkliste von Derogatis —Deutsche Version. Göttingen (Germany): Beltz Test GMBH; 2002. SCL-90-R. [Google Scholar]
- 40.Hellweg R, von Arnim CA, Buchner M, et al. Neuroprotection and neuronal dysfunction upon repetitive inhibition of oxidative phosphorylation. Exp Neurol. 2003;183:346–54. doi: 10.1016/s0014-4886(03)00127-4. [DOI] [PubMed] [Google Scholar]
- 41.Ziegenhorn AA, Schulte-Herbruggen O, Danker-Hopfe H, et al. Serum neurotrophins — a study on the time course and influencing factors in a large old age sample. Neurobiol Aging. 2007;28:1436–45. doi: 10.1016/j.neurobiolaging.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Franke L, Schewe HJ, Muller B, et al. Serotonergic platelet variables in unmedicated patients suffering from major depression and healthy subjects: relationship between 5HT content and 5HT uptake. Life Sci. 2000;67:301–5. doi: 10.1016/s0024-3205(00)00620-2. [DOI] [PubMed] [Google Scholar]
- 43.Pan W, Banks WA, Fasold MB, et al. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–61. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 44.Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328:261–4. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- 45.Lang UE, Hellweg R, Seifert F, et al. Correlation between serum brain-derived neurotrophic factor level and an in vivo marker of cortical integrity. Biol Psychiatry. 2007;62:530–5. doi: 10.1016/j.biopsych.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Moffett JR, Ross B, Arun P, et al. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lommatzsch M, Zingler D, Schuhbaeck K, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–23. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Trajkovska V, Marcussen AB, Vinberg M, et al. Measurements of brain-derived neurotrophic factor: methodological aspects and demographical data. Brain Res Bull. 2007;73:143–9. doi: 10.1016/j.brainresbull.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 49.Mercader JM, Fernandez-Aranda F, Gratacos M, et al. Blood levels of brain-derived neurotrophic factor correlate with several psychopathological symptoms in anorexia nervosa patients. Neuropsychobiology. 2007;56:185–90. doi: 10.1159/000120623. [DOI] [PubMed] [Google Scholar]
- 50.Mamounas LA, Blue ME, Siuciak JA, et al. Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J Neurosci. 1995;15:7929–39. doi: 10.1523/JNEUROSCI.15-12-07929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Djalali S, Holtje M, Grosse G, et al. Effects of brain-derived neurotrophic factor (BDNF) on glial cells and serotonergic neurones during development. J Neurochem. 2005;92:616–27. doi: 10.1111/j.1471-4159.2004.02911.x. [DOI] [PubMed] [Google Scholar]
- 52.Mamounas LA, Altar CA, Blue ME, et al. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000;20:771–82. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lang UE, Hellweg R, Gallinat J. Association of BDNF serum concentrations with central serotonergic activity: evidence from auditory signal processing. Neuropsychopharmacology. 2005;30:1148–53. doi: 10.1038/sj.npp.1300666. [DOI] [PubMed] [Google Scholar]
- 54.Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;59:673–80. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 55.Wichers M, Kenis G, Jacobs N, et al. The BDNF Val(66)Met × 5-HT-TLPR × child adversity interaction and depressive symptoms: an attempt at replication. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:120–3. doi: 10.1002/ajmg.b.30576. [DOI] [PubMed] [Google Scholar]