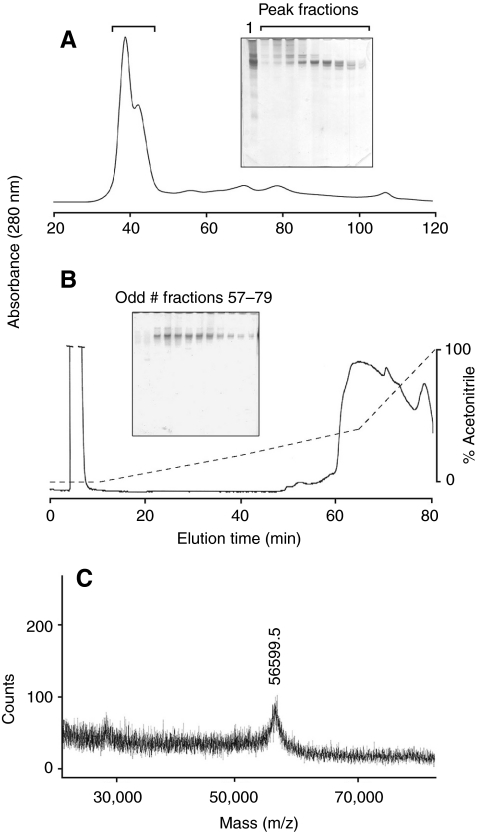
Fig. 2.
Partial purification of thread matrix proteins (TMPs). (A) Size exclusion chromatography of acid extractable proteins from the distal thread. Proteins were extracted from distal threads with 5% acetic acid and separated by gel filtration on Sephacryl S-200. The solid line is the absorbance at 280 nm. The proteins in the fractions under the bracket are shown separated on a 10% acid–urea polyacrylamide gel electrophoresis (AU PAGE) gel. Lane 1 shows crude extracts. (B) Reverse-phase-high performance liquid chromatography (RP-HPLC) separation of TMPs. Fractions containing significant amounts of the TMPs were separated on a C4 RP-HPLC column. Solid line is the absorbance at 280 nm. The broken line is the gradient of acetonitrile. The TMPs eluted as a broad indistinct peak. Odd fractions between 57 and 79 min are shown separated on a 10% AU PAGE gel. (C) Matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectrometry of partially purified TMPs. The only proteins to desorb and ionize exhibited a [M + H]1+ of 56.5 kDa.