Abstract
Purpose
The purpose of this open- label phase II SWOG study was to evaluate the activity of gemcitabine (Gemzar ®; Eli Lilly, Indiana, USA) and cisplatin combination therapy, in patients with unresectable malignant mesothelioma of the pleura.
Patients and methods
Fifty eligible chemotherapy naïve patients with histologically proven malignant mesothelioma of the pleura, and a SWOG performance status 0–2 were enrolled between February 1999 to August 2000. Treatment consisted of gemcitabine 1000mg/m2 and cisplatin 30 mg/m2 on days 1,8 and 15 of a 28-day cycle, until progression of disease or two cycles beyond complete response.
Results
Using SWOG response criteria, one patient had a confirmed complete response and five patients had a confirmed partial response, for a total response rate of 12% (95% C.I. of 5% – 24%). All the responses were seen in patients with epithelioid or unspecified histology. Stable disease was seen in 25 patients (50%). The median overall survival was 10 months (95% C.I. 7 – 15 mo.), with a median progression free survival of 6 months. Sixteen patients experienced Grade 4 toxicity. Twelve of these grade 4 toxicities were hematologic. There were no treatment-related deaths.
Conclusions
Cisplatin-gemcitabine combination chemotherapy has modest activity with an acceptable toxicity profile, as first line treatment for patients with malignant mesothelioma.
Keywords: Malignant Mesothelioma, Cisplatin, Gemcitabine, Chemotherapy, Phase II
Introduction
Malignant pleural mesothelioma (MPM) is an uncommon, neoplastic disorder of the pleural lining of the lung, usually presenting at an advanced stage and generally considered resistant to conventional chemotherapy treatment. The majority of cases (60–80%) occur in male patients, and are attributed to asbestos exposure, with a long latency between exposure and presentation (1, 2). Although the incidence of the disease may have peaked in the United States, it continues to rise throughout much of the world where it is not expected to peak until sometime between 2010 and 2020 (3, 4).
The median survival of patients with unresectable MPM averages approximately 12 months (5, 6). Numerous chemotherapy agents have been tested in phase II trials. Single agent chemotherapy has generally yielded response rates between 0–20%. Combination chemotherapy has resulted in somewhat higher response rates of 10–40%(7). An impact on survival has been difficult to demonstrate until the recent phase III trials using cisplatin and the newer antifolates (i.e., pemetrexed and raltitrexed) which have now demonstrated a significant improvement in response rate and a survival advantage compared to cisplatin alone (5, 6).
In a murine mesothelioma model, gemcitabine has shown additive anti-tumor effects when administered in combination with cisplatin(8). This combination was initially studied in Australia with promising results in MPM (9, 10). In these studies cisplatin was administered at a dose of 100 mg/m2 on day 1, with gemcitabine at 1,000 mg/m2 on days 1, 8 and 15 of a 28-day cycle. This Southwest Oncology Group (SWOG) study was designed to confirm and extend these results by using the same dose of gemcitabine, but dividing the cisplatin into three weekly doses to reduce the toxicity. The additional hypothesis was that this weekly dosing regimen might make allow greater synergism between the two agents.
Patients and Methods
Between February 1999 and August 2000, 57 patients with unresectable MPM were enrolled onto this study at participating institutions from the SWOG. Patients were required to have histologically confirmed MPM of the pleura with bidimensionally measurable disease, SWOG performance status 0–2, and no prior chemotherapy or radiotherapy for any reason. Patients may have undergone prior surgery at least four weeks before study enrollment, and should have recovered from all side effects associated with surgery.
Adequate bone marrow function (total leukocyte count ≥ 3,000,/μl, and platelet count ≥ the institutional lower limit of normal), hepatic function (bilirubin ≤ 2 mg/dl and aspartate aminotransferase or alanine aminotransferase ≤ 2.5 times the institutional upper limit of normal) and renal function (estimated creatinine clearance ≥ 60 ml/min, and serum creatinine ≤ twice the institutional upper limit of normal) were required. Patients with prior malignancies (other than non-melanoma skin cancer, and adequately treated cervical cancer) were excluded if the disease free interval from the other malignancy was less than five years. Pregnant or nursing mothers were excluded. Patients with reproductive potential were required to use an effective contraceptive method. This study was approved by each individual institutional review board, and written informed consent was obtained from each patient in accordance with institutional and federal guidelines.
All patients received gemcitabine 1,000 mg/m2 over 30 minutes and cisplatin 30 mg/m2 over 30 minutes on days 1,8 and 15 of a 28-day cycle. An appropriate anti-emetic regimen was administered according to the institutional standards of care.
Dose adjustments
The study used NCI Common Toxicity Criteria version 2.x for toxicity and adverse event reporting. NCI CTC (National Cancer Institute Common Toxicity Criteria) Grade 3 non-hematological toxicity required a 50% reduction of the doses of both drugs, or withholding the doses based on the judgement of the treating investigator. NCI CTC Grade 4 non-hematological toxicity required withholding the medications till resolution of such toxicity, and a reinitiation of chemotherapy with a 50% dose reduction. Grade 2 diarrhea or mucositis required the gemcitabine dose to be withheld, and grade 3 or 4 diarrhea or mucositis required withholding the dose and a 50% reduction of the subsequent doses.
The gemcitabine dose was reduced by 25% for an absolute neutrophil count (ANC) less than 1,500/μl or a platelet count less than 100,000/μl. It was reduced by 50% for an ANC less than 1,250/μl or a platelet count less than 75,000/μl. Gemcitabine was withheld if the ANC was less than 1,000/μl or a platelet count less than 50,000/μl. In the event of a dose being held, because of cytopenias, the drug could be reinitiated with a 25% reduction for all subsequent cycles.
Cisplatin dose modification was performed on the day of treatment, and was based on the estimated creatinine clearance. If the estimated creatinine clearance was less than 60 ml/min, a 50% dose reduction was required. If the creatinine clearance decreased to less than 40ml/min, cisplatin was discontinued. Grade 2 peripheral neuropathy required a 50% dose reduction of cisplatin and grade 3 peripheral neuropathy required the discontinuation of cisplatin. Patients requiring discontinuation of cisplatin due to the above toxicities were continued on single agent gemcitabine.
Growth factor support with granulocyte colony stimulating factor, (G-CSF Neupogen ®; Amgen, California) was not permitted in the first cycle. However, it could be used for patients who developed grade 3 or 4 neutropenia, or neutropenic fever, for all subsequent cycles of chemotherapy. If an adequate neutrophil count was maintained with the initial cycle of G-CSF supported chemotherapy, dose escalation to the original dose level with subsequent cycles of chemotherapy was allowed.
Response assessment
All patients entered into the study underwent a baseline clinical history, physical examination, laboratory evaluation, CT scan of the chest, chest x-ray, and an audiogram. Patient examination and laboratory evaluation was performed weekly prior to each treatment. Staging studies were repeated after every two cycles for tumor assessment.
Tumor response was defined as complete response, partial response, stable disease or progression according to the SWOG criteria(11). Complete response (CR) was defined as the disappearance of all measurable and evaluable disease. Partial response (PR) was defined as a 50% or greater decrease in the sum of the products of the perpendicular diameters of all measurable lesions. Stable disease (SD) was defined as a response which did not qualify for complete response, partial response or progression. Progressive disease was defined as a 50% increase or an increase of 10cm2 (whichever was smaller) in the sum of products of all measurable lesions over the smallest observed (over baseline if no decrease), clear worsening of any evaluable disease, reappearance of any lesion which had disappeared, appearance of a new lesion, or failure to return for evaluation due to death or deteriorating condition. All measurable, evaluable and non-evaluable lesions were assessed using the same technique (CT scan, MRI, Plain X-ray or palpable lesion greater than 2 cm.) as baseline. All responses were confirmed a minimum of three weeks later with the same imaging modality. Patients were removed from the protocol treatment if they experienced unacceptable toxicity, disease progression, delay in treatment of greater than three weeks due to toxicity, or if they required radiation to any site for symptom relief, withdrew consent, or completed two cycles after complete response. Responding patients were continued on chemotherapy till disease progression or undue toxicity. In patients with a complete response, chemotherapy was given for two cycles beyond best response.
Statistics
The primary endpoint of this study was the median overall survival calculated from study entry date. Accrual of 50 patients was required to allow for assessment of 1-year survival to within ±14% of actual survival (95% C.I). Response rates and rates of specific toxicities could also be estimated to within ± 14% (95% C.I.). Any toxicity with at least a 5% probability was likely to be seen at least once (92% chance)
Results
Fifty-seven patients were registered on this study. Seven patients were found to be ineligible due to insufficient documentation of disease (2 patients), inadequate baseline hematologic or renal function (3 patients) and no measurable disease (2 patients). Eleven patients were removed from protocol therapy before progression of disease, completion of treatment, or toxicity for reasons not specified in the protocol. This included worsening of disease not qualifying as disease progression per protocol (3 patients), lack of benefit (or further benefit; 4 patients), physicians stopping treatment in error or for undocumented reasons (2 patients), non-compliance (1 patient) and grand mal seizures unrelated to protocol treatment (1 patient). Treatment for one patient was stopped after 4 weeks in error, then re-started again after a six week delay and was coded as a major protocol deviation. Patient characteristics are detailed in Table 1.
Table 1.
Patient demographics and baseline characteristics
| Number | Per cent | |
|---|---|---|
| Sex | ||
| Male | 44 | 88% |
| Female | 6 | 12% |
|
| ||
| Age, years | ||
| Median | 69 | |
| Range | 36–80 | |
|
| ||
| Race | ||
| Caucasian | 46 | 92% |
| African American | 1 | 2% |
| Asian | 2 | 4% |
| Native American | 1 | 2% |
|
| ||
| Histopathology | ||
| Epithelioid | 25 | 50% |
| Sarcomatoid | 4 | 8% |
| Mixed | 3 | 6% |
| Not specified | 18 | 36% |
|
| ||
| SWOG Performance status | ||
| 0 | 13 | 26% |
| 1 | 27 | 54% |
| 2 | 10 | 20% |
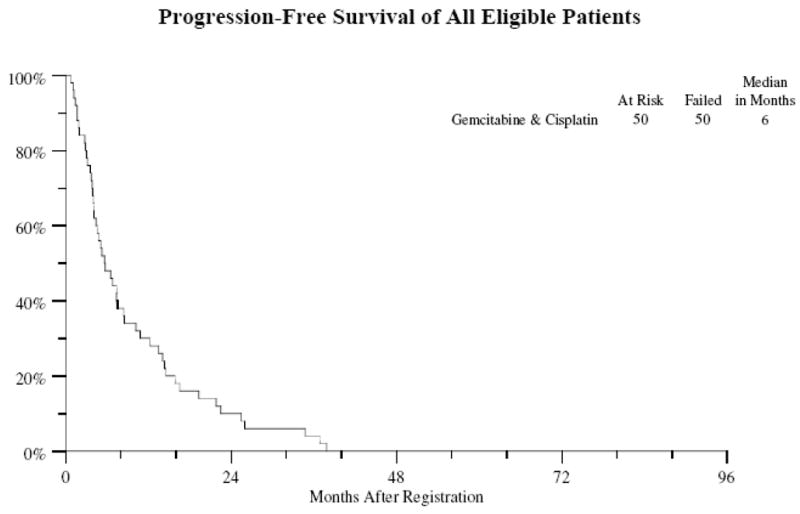
Of the 50 eligible patients 48 patients have died. The median survival is 10 months (Figure 1) (95% CI 7–15 months). All 50 patients have either progressed or died in absence of progression with a median progression-free survival of 6 months (Figure 2) (95% CI 4–8 months). Survival at 1-year was 30%.
Figure 1.
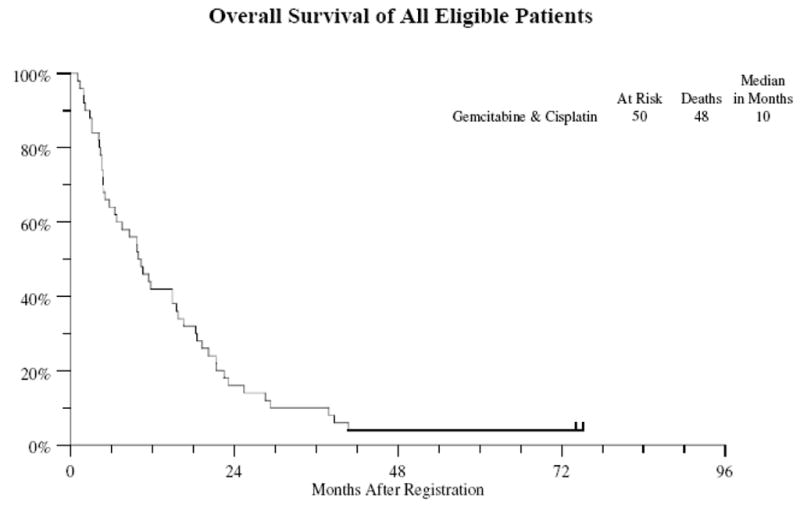
Figure 2.
Based on an intention to treat analysis, of the 50 eligible patients, 1 patient (2%; 95% confidence interval 0% to 11%) had a confirmed complete response. Five other patients had confirmed partial responses (10%; 95% CI 3–22%). The overall response rate was thus 12% (95% CI 5–24%). Since 12 patients could not be assessed for response, of the patients assessable for response, the response rate was 16% (6/38). All the responses were seen in patients with epithelioid or unspecified histology. The responses classified by histology are outlined in table 2. Patients received a median of 3 cycles of treatment (range 1–8 cycles).
Table 2.
response classified by histology
| Response | Epithelioid n=25 | Sarcomatoid n=4 | Mixed n=3 | Unspecified n=18 |
|---|---|---|---|---|
| Complete | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Partial | 3 (12%) | 0 (0%) | 0 (0%) | 2 (11%) |
| Stable disease | 12 (48%) | 3 (75%) | 2 (67%) | 8 (44%) |
| Progression of disease | 3 (12%) | 1 (25%) | 0 (0%) | 3 (17%) |
| Inadequate assessment | 6 (24%) | 0 (0%) | 1 (33%) | 5 (28%) |
Toxicity
The 50 eligible patients have been assessed for toxicity. There were 17 grade 4 toxicities in 16 patients. Twelve patients experienced Grade 4 hematologic toxicities, and five patients had non-hematologic Grade 4 toxicities. There were no treatment-related deaths. Grade 3& 4 toxicities are outlined in table 3.
Table 3.
Most commonly observed toxicities
| Toxicity | Grade 3: No. pts (%) | Grade 4: No. pts (%) |
|---|---|---|
| Hematological | ||
| Anemia | 10 (20%) | 2 (4%) |
| Leukopenia | 14 (28%) | 1 (2%) |
| Febrile neutropenia | 1 (2%) | 0 (0%) |
| Neutropenia | 15 (30%) | 10 (20%) |
| Red blood cell transfusion | 15 (30%) | 0 (0%) |
| Thrombocytopenia | 16 (32%) | 0 (0%) |
| Non-Hematological | ||
| ARDS | 0 (0%) | 1 (2%) |
| Constipation | 2 (4%) | 0 (0%) |
| Cough | 3 (6%) | 0 (0%) |
| Creatinine increase | 0 (0%) | 1 (2%) |
| Dehydration | 2 (4%) | 0 (0%) |
| Dyspnea | 8 (16%) | 2 (4%) |
| Fatigue/Malaise | 11 (22%) | 1 (2%) |
| Hypersensitivity reactions | 1 (2%) | 0 (0%) |
| Infections | 3 (6%) | 0 (0%) |
| Inner ear hearing loss | 2 (4%) | 0 (0%) |
| Motor neuropathy | 1 (2%) | 0 (0%) |
| Muscle weakness | 3 (6%) | 0 (0%) |
| Nausea | 3 (6%) | 0 (0%) |
| Sensory neuropathy | 1 (2%) | 0 (0%) |
| Somnolence | 2 (4%) | 0 (0%) |
Discussion
This multicenter cooperative group study has failed to confirm the response rate seen with this combination in two prior trials in Australia (9, 10). Although the response rates were lower, the median survival appears to be equivalent to the other trials. In the first single institution study by Byrne et al, 10 of the 21 enrolled patients (47%) exhibited a partial response. Nine of the 10 patients had epithelioid mesothelioma, and one patient had a mixed histology. The estimated median progression free survival was 25 weeks and the estimated median overall survival was 41 weeks (10 months). In the subsequent multicenter study led by Nowak et al, 17 of the 52 assessable patients (33%) exhibited a partial response. The median time to disease progression was 6.4 months, and the median overall survival was 11.2 months. In these trials, cisplatin was given as a single dose on day 1 rather than the weekly dosing schedule as in the current trial. The dose of Cisplatin was also slightly higher at 100 mg/m2 per cycle rather than the 90 mg/m2 used in this trial. Lower response rates have been seen in other trials, which have employed this combination with a lower dose of cisplatin. In a study performed in the Netherlands, cisplatin was given at 80 mg/m2 along with gemcitabine 1,250 mg/m2 every 21 days(12). Four partial responses were seen in 25 patients (16%). The time to progression was 6 months (5–7 months) with a median survival of 9.6 months. In a recently completed ECOG study, with cisplatin 75 mg/m2 along with gemcitabine 1,250 mg/m2 given on day 1 and 8, partial responses were seen in nine of the 26 patients (26%)(13). The median progression free survival was 8 months and the median survival was 13 months.
It is very hard to compare response rates across these studies. In our study, the responses were assessed using the bidimensional measurements as per SWOG criteria, while other studies used modified RECIST criteria(14). Measurement of mesothelioma in the bidimensional fashion is difficult which has led to the adoption of the pleural rind measurements in the new criteria. The lower response rate seen in the current study could also be attributed to the lower dose of cisplatin employed in this regimen. The frequencies and severity of toxicities experienced with this regimen appear to be comparable to other regimens in this patient population. The regimen was well tolerated with no toxic deaths.
Historically, survival in malignant mesothelioma without systemic chemotherapy has been in the range of 6–9 months(15–17). Recent randomized phase III trials with combinations of antifolate with cisplatin have demonstrated a survival advantage with this combination as compared to single agent cisplatin. In the trial comparing cisplatin-pemetrexed to cisplatin alone, the response rate with the combination was 41% in comparison to 17% with cisplatin. The median progression free survival improved significantly from 3.9 to 5.7 months (p=0.001). The median survival improved from 9.3 months to 12.1 months (p=0.02). In a similar randomized phase III trial conducted with raltitrexed and cisplatin in Europe, the response rate was 23%, with a median survival of 11.4 months.
In conclusion, the cisplatin-gemcitabine combination has modest activity with an acceptable toxicity profile, as frontline treatment of patients with malignant mesothelioma. With the recent demonstration of a survival advantage in two phase III studies utilizing the combination of cisplatin with an antifolate, this combination of cisplatin-gemcitabine is not being further studied by the SWOG for patients with advanced malignant pleural mesothelioma.
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA38926, CA32102, CA14028, CA35192, CA42777, CA20319, CA46441, CA35176, CA35128, CA67575, CA45807, CA35178, CA67663, CA63848, CA12213, CA58882, CA35262, CA35431, and supported in part by Eli Lilly and Company. Dr. Petrylak receives research funding for his clinical research from Eli Lilly and company.
Footnotes
(Note to Editor: Please DO NOT send editorial correspondence or proofs to reprint address.)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antman KH. Natural history and epidemiology of malignant mesothelioma. Chest. 1993;103(4 Suppl):373S–6S. doi: 10.1378/chest.103.4_supplement.373s. [DOI] [PubMed] [Google Scholar]
- 2.Zellos L, Christiani DC. Epidemiology, biologic behavior, and natural history of mesothelioma. Thorac Surg Clin. 2004;14(4):469–77. viii. doi: 10.1016/j.thorsurg.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Peto J, Decarli A, La Vecchia C, Levi F, Negri E. The European mesothelioma epidemic. Br J Cancer. 1999;79(3–4):666–72. doi: 10.1038/sj.bjc.6690105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price B. Analysis of current trends in United States mesothelioma incidence. Am J Epidemiol. 1997;145(3):211–8. doi: 10.1093/oxfordjournals.aje.a009093. [DOI] [PubMed] [Google Scholar]
- 5.van Meerbeeck JP, Gaafar R, Manegold C, et al. Randomized Phase III Study of Cisplatin With or Without Raltitrexed in Patients With Malignant Pleural Mesothelioma: An Intergroup Study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada 10.1200/JCO.20005.14.589. J Clin Oncol. 2005;23(28):6881–9. doi: 10.1200/JCO.20005.14.589. [DOI] [PubMed] [Google Scholar]
- 6.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III Study of Pemetrexed in Combination With Cisplatin Versus Cisplatin Alone in Patients With Malignant Pleural Mesothelioma 10.1200/JCO.2003.11.136. J Clin Oncol. 2003;21(14):2636–44. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 7.Steele JP, Klabatsa A. Chemotherapy options and new advances in malignant pleural mesothelioma. Ann Oncol. 2005;16(3):345–51. doi: 10.1093/annonc/mdi094. [DOI] [PubMed] [Google Scholar]
- 8.Davidson JA, Robinson BWS. Gemcitabine activity on murine and human malignant mesothelioma cell lines show additive activity in combination with cisplatin. Aust N Z J Med. 1997;27:213. [Google Scholar]
- 9.Byrne MJ, Davidson JA, Musk AW, et al. Cisplatin and Gemcitabine Treatment for Malignant Mesothelioma: A Phase II Study. J Clin Oncol. 1999;17(1):25. doi: 10.1200/JCO.1999.17.1.25. [DOI] [PubMed] [Google Scholar]
- 10.Nowak AK, Byrne MJ, Williamson R, et al. A multicentre phase II study of cisplatin and gemcitabine for malignant mesothelioma. Br J Cancer. 2002;87(5):491–6. doi: 10.1038/sj.bjc.6600505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green S, Weiss GR. Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Invest New Drugs. 1992;10(4):239–53. doi: 10.1007/BF00944177. [DOI] [PubMed] [Google Scholar]
- 12.van Haarst JM, Baas P, Manegold C, et al. Multicentre phase II study of gemcitabine and cisplatin in malignant pleural mesothelioma. Br J Cancer. 2002;86(3):342–5. doi: 10.1038/sj.bjc.6600118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castagneto B, Zai S, Dongiovanni D, et al. Cisplatin and gemcitabine in malignant pleural mesothelioma: a phase II study. Am J Clin Oncol. 2005;28(3):223–6. doi: 10.1097/01.coc.0000144852.75613.56. [DOI] [PubMed] [Google Scholar]
- 14.Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15(2):257–60. doi: 10.1093/annonc/mdh059. [DOI] [PubMed] [Google Scholar]
- 15.Tomek S, Emri S, Krejcy K, Manegold C. Chemotherapy for malignant pleural mesothelioma: past results and recent developments. Br J Cancer. 2003;88(2):167–74. doi: 10.1038/sj.bjc.6600673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berghmans T, Paesmans M, Lalami Y, et al. Activity of chemotherapy and immunotherapy on malignant mesothelioma: a systematic review of the literature with meta-analysis. Lung Cancer. 2002;38(2):111–21. doi: 10.1016/s0169-5002(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 17.Ong S, Vogelzang N. Chemotherapy in malignant pleural mesothelioma. A review. J Clin Oncol. 1996;14(3):1007–17. doi: 10.1200/JCO.1996.14.3.1007. [DOI] [PubMed] [Google Scholar]


