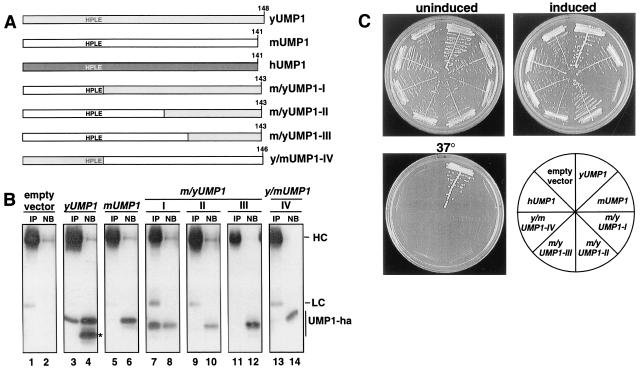
Figure 2.
Mammalian and yeast UMP1 proteins are not functionally interchangeable. (A) Schematic representation of constructs. (B) Coimmunoprecipitation assay to detect association of various Ump1-ha variants with proteasomal complexes. Proteins were precipitated from extracts of the same strains shown in C (except for those expressing hUMP1) with antibodies against yeast 20S proteasomes. The precipitates (IP) as well as 25% of the nonbinding material (NB) were analyzed by Western blotting with anti-ha antibody. The positions of IgG heavy chains (HC), light chains (LC), and various Ump1-ha proteins are indicated. The position of a product of yUMP1, missing the domain necessary for interaction with the proteasome, is marked with an asterisk. (C) Growth assays of yeast strain JD59 (ump1-Δ) transformed with high-copy plasmids expressing, from the copper-inducible PCUP1 promoter, human (h), mouse (m), or yeast (y) UMP1 genes or various chimeric genes of the latter two (m/yUMP1-I to m/yUMP1-III or y/mUMP1 depicted in A; see Materials and Methods and main text for details). Transformants were streaked onto selective minimal medium, with (induced) or without (uninduced) 100 μM CuSO4, and incubated at 30°C for 3 days. To assay for complementation of ump1-Δ temperature-sensitive growth phenotype, transformants were streaked onto complete medium and incubated at 37°C for 2 days.