Abstract
Transcutaneous immunization aims at taking advantage of the skin’s immune system for the purpose of immunoprotection. In the present study we evaluated the potential of topical delivery of a recombinant melanoma protein, HR-gp100, derived from a shorter sequence of the native gp100 gene. The protein was applied on the skin, with and without the addition of two forms of heat labile enterotoxin (nLT and LTB). HR-gp100 fused to Haptide, a cell penetrating 20mer peptide (HR-gp100H) was also tested. Topical HR-gp100 and HR-gp100H application on the ears of mice elicited the production of specific antibodies, and transcutaneous delivery to intact human skin induced dose-dependent LC activation. nLT and LTB also activated LC, but did not further increase the activation induced by HR-gp100. These results show that HR-gp100, an antigenic tumor-derived protein, activates the immune system following transcutaneous delivery, as shown by both Langerhans cell activation and induction of antibody production.
Keywords: melanoma, transcutaneous vaccination, Langerhans cells
1. Introduction
Transcutaneous immunization aims at taking advantage of the skin’s immune system for the purpose of immunoprotection or immunomodulation [1]. The skin is populated by densely-distributed and potent antigen presenting cells, Langerhans’ cells (LC) and dermal dendritic cells. In vitro-generated equivalents of these cells, such as human monocyte-derived dendritic cells (hDC), are being evaluated actively in cancer immunotherapy [2]. Topical antigen delivery for cancer immunotherapy would have practical advantages as well as immunological merits, since it would allow to load and activate DC in their physiological milieu.
Microbial antigenic products act as danger signals that have been shown to activate adaptive immunity. Among them, E. coli heat-labile enterotoxin (LT) has been shown to act as a safe and effective adjuvant for the induction of humoral and cellular responses, including cytotoxic T cell responses (CTL) [3–5], and for transcutaneous antigen administration in mice [6] and in humans [7]. We have recently shown that a non-toxic mutant, nLT, and its B subunit (LTB), are taken up by dendritic cells, and induce their maturation and activation. When delivered topically, nLT and LTB induce production of antibodies [8].
The use of whole protein for DC loading has several major advantages over the use of peptides. Proteins are taken up endogenously, are HLA-independent, may contain several antigenic epitopes, and may stimulate both CD4+ and CD8+ T cells. We have recently produced hydrophilic recombinant gp100 (HR-gp100), a modified version of the melanoma protein gp100 that is known to contain several immunogenic peptide antigens, and shown it to be effectively taken up by DC and presented to CD8+ lymphocytes [9,10]. Application of HR-gp100 to the skin of mice elicited production of anti-HR-gp100 antibodies [8].
A recombinant version of HR-gp100 that contains homologous sequences of the new short fibrin 20mer peptide, homologous to the c-terminal chain of fibrinogen, (preCγ) was produced in order to improve its penetration into cells. This peptide is one of a newly described family of cell binding and penetrating 20-mer fibrinopeptides (called Haptides) [11] that were also shown to improve entry of nanoparticles such as liposomes into cells [12]. HR-gp100 with the Haptide preCγ sequence (HR-gp100H) was prepared with the intent that the Haptide would improve the cell penetration and subsequent presentation of the antigen.
The study of human skin in vivo (i.e. on volunteers) is limited for ethical and medical reasons, and skin in animals is significantly different from human skin. Organ culture of human skin has been performed successfully by a few groups, and used in various dermatological studies, including activation of LC [13–15]. However, in the organ culture system, nutrients are supplied to the skin by diffusion of culture medium through the entire tissue, and not by perfusion of blood.
Although the epidermis remains vital for several days in organ culture, the thickness of the epidermis slowly diminishes, and dermal blood vessels and other structures degenerate. Transplantation of human skin to SCID mice has been used as a dermatological model that more closely mimics the in-vivo physiology of the skin, but these mice are extremely expensive, and their immunodeficiency makes them very susceptible to disease.
An alternative system for study of human skin is transplantation to the highly vascularized chorioallantoic membrane (CAM) of the chicken egg. The chick CAM has successfully been used for studies of angiogenesis and anti-angiogenesis in the development of anti-cancer treatments. It has the advantages of simplicity, very low cost, and easy availability. The chick CAM has also been shown to support the survival of human skin [16], and LC have been shown to be retained in the skin several days after grafting to the CAM [17].
We have used the CAM culture technique to grow intact human skin up to 10 days, and demonstrated significant improvements in vitality over organ culture, and have used the system to evaluate chemical irritation and sensitization (Agassi et al, manuscript in preparation).
In the present study, we further evaluated the potential of topically delivered HR-gp100, and its preCγ Haptide containing derivative with and without the addition of heat labile enterotoxin to activate LC and to induce immune responses. LC activation was evaluated in intact human skin using both traditional organ culture and the chicken CAM models, and stimulation of immune responses in vivo was examined in BALB/c mice.
2. Materials and Methods
2.1 Mutated non-toxic LT (nLT)
The LT protein whose gene (accession number AB011677) bears two point mutations in the A1 subunit (tryptophan 50 and valine 53 both changed to proline) to reduce its toxicity [10] was kindly provided by Gavish (Glil Yam, Israel). LTB was produced as described by Fingerut et al [18]. Briefly, the B part of the nLT gene was isolated by PCR and cloned into Pichia pastoris yeast cells. Following protein expression induced by methanol, LTB was purified and concentrated by cation exchange chromatography.
2.2 HR-gp100 and HR-gp100-Haptide (HR-gp100H)
Hydrophobic recombinant gp100 (HR-gp100) was prepared in bacteria [9] and in yeast [10]. HR-gp100 with Haptide preCγ sequence (TRWYSMKKTTMKIIPFNRL) [11] added to the C′ terminal was cloned and expressed in E. coli using the method previously described for cloning of HR-gp100 [9].
2.3 Transcutaneous antigen delivery in mice
Mice were treated as previously described [8]. Briefly, BALB/c mice were anesthetized with ketamine and xylasine (9:1). The ears were cleaned with 70% ethanol, and HR-gp100 or HR-gp100H in PBS was applied on the ventral and dorsal sides of the ear, either alone or mixed with nLT or LTB. The molecules were applied three times at two-week intervals. The day before each application a few drops of blood were drawn from the tail of the mice for the determination of antibody levels. Anti-LT antibodies were determined by ELISA, as previously described [9]. Briefly, plates were incubated with GM1 receptor (monosialoganglioside-GM1, Sigma). Following blocking, plates were incubated with commercial cholera toxin (CT, Sigma). After incubation with the mouse sera, goat anti-mouse IgG-conjugated HRP (Sigma) was added, followed by the substrate o-Phenylenediamine dihydrochloride. Optical density was determined using an ELISA reader at OD450nm. Anti-HR-gp100 antibodies were determined as described by Gelbart et al [9]. Briefly, plates were coated with chicken anti HR-gp100, followed by blocking and incubation with HR-gp100. Mouse sera were added, and the assay was continued as above.
2.4 Human Skin-CAM grafts
The technique of Kunzi-Rapp et al [17] for grafting human skin to the chicken CAM was used with minor modifications. Briefly, 6mm diameter punch biopsies of full-thickness intact human skin from mastectomies were used throughout this study. Excess subcutaneous fat was removed before grafting. The CAM of 7–9 day-old chick embryos was mildly abraded near a junction of large blood vessels, and skin was then placed on the CAM with the dermal side in contact with the membrane. Excess subcutaneous fat was removed before grafting. Skin was always transplanted within 4 hours of surgical removal.
2.5 Human Skin-organ culture
The method used was adapted from widely used organ culture techniques [19]. Skin samples, about 6 mm diameter, were obtained as in 2.4 above. The explants were placed on polyester meshes (mesh size: 74 μm, Costar® Netwells). Minimum Essential Medium with 10% Fetal Calf Serum and Penicillin-Sterptomycin-Amphotericin B solution was used. The epidermis was exposed to air to mimic natural skin condition.
2.5.1 Exposure of human skin to immunostimulatory proteins
A plastic ring cut from an Eppendorf pipettor tip was placed on top of the skin in the organ culture wells or the skin grafted 4 days previously to the CAM. A 100μl drop of a known sensitizer, 2,4-dinitrofluorobenzene (DNFB, dissolved in acetone: olive-oil 4:1), or solutions of HR-gp100, HR-gp100H, nLT, LTB, or bovine serum albumin in PBS were placed in the ring. After an exposure of 12–18h to the solutions, the skin was fixed in 4% paraformaldehyde and embedded in paraffin, and 6μ sections were stained with either hematoxylin and eosin or with a monoclonal antibody to the Langerhans cell marker CD1a (Santa Cruz Biotechnology). CD1a+ cells were detected with peroxidase-coupled secondary antibodies and AEC. The decrease in CD1a+ cells in the epidermis is a direct measure of their migration [20]. Staining was also performed for the dendritic cell maturation marker CD83 using a monoclonal antibody (Clone HB15a, Santa Cruz Biotechnology) with a fluorescent tag. None of the applied reagents caused obvious epidermal cell death, as determined by lack of pycnotic nuclei with Hoechst staining or changes in epidermal keratinocyte proliferation as quantified by Ki67 staining (not shown).
2.5.2 Data Analysis
In experiments using human skin, at least 4 fragments of skin from the same donor were used for each treatment. CD1a+ LC in the epidermis were counted in two sections from one slide for each skin fragment, and an average was made of the 8 counts for each treatment. The area of the epidermis in the analyzed sections was determined using ImageJ software (rsb.info.nih.gov/ij/), and the results expressed as CD1a+ LC/mm2 of epidermis.
2.6 Statistical evaluation
The two-tailed Student’s t-test was used. Each experiment was performed at least twice (with the exception of the CD83 immunostaining). Error bars indicate the standard error or the standard deviation of the mean, as stated.
3. Results
3.1 Transcutaneous delivery of HR-gp100 elicits antibody production
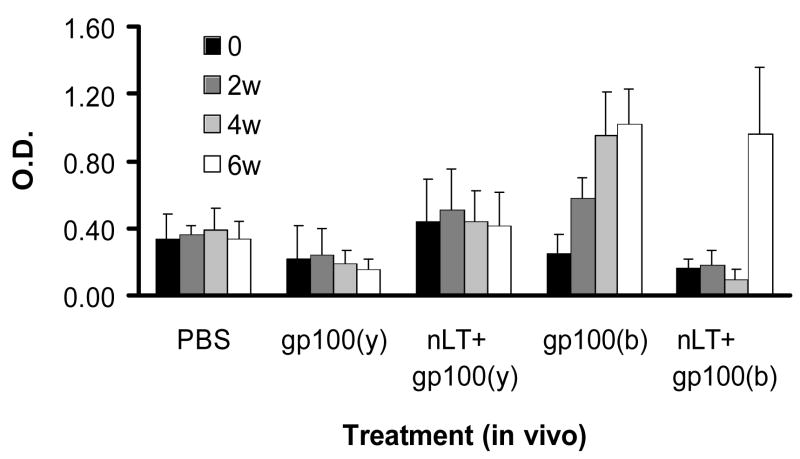
Purified recombinant HR-gp100 produced in yeast or bacteria was applied to the ears of mice either alone or mixed with nLT. Bacterial HR-gp100 induced steadily increasing production of antibodies, which was significant (p<0.05) after the third protein application. By contrast, transcutaneous administration of HR-gp100 produced in yeast did not induce antibody production, and application of nLT did not further enhance antibody levels produced by HR-gp100 (Fig. 1). Furthermore, nLT seemed to somewhat inhibit the levels of anti-gp100 antibodies. This effect, although not statistically significant, was observed in every experiment, and was also found in mice treated with LTB. In fact, the level of anti-CT antibodies in mice treated with LTB+HR-gp100 was comparable to the level of anti-CT antibodies in mice treated with LTB alone (not shown).
Figure 1.
Serum anti-HR-gp100 antibody levels in mice treated topically with 50–100 μg/mouse HR-gp100 produced in yeast, gp100(y), or bacteria, gp100(b), either alone or mixed with nLT. Each treatment was repeated 2 or 3 times in independent experiments; the results of one typical experiment are shown. w – weeks after starting topical treatment. Each group included 3 mice; bars indicate standard error of the means.
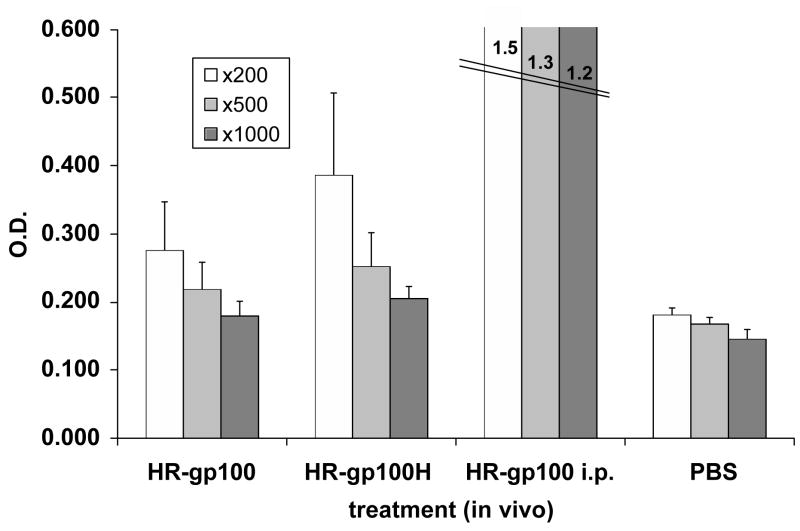
In further experiments, mice were treated topically with HR-gp100 or with its Haptide-containing sequence HR-gp100H. The results, shown in Fig. 2, reveal a significant increase in antibody titer in the serum of mice treated with HR-gp100 at dilutions of 1:200, 1:500 and 1:1000 (p< 0.02), and an even greater increase in antibodies against HR-gp100H, compared to PBS-treated mice, applied at the same dilutions (p<0.0002-p<0.008). The antibody levels in mice treated with HR- gp100H were significantly higher than in mice treated with HR-gp100 at the 1:200 (p<0.04) and the 1:1000 (p<0.02) dilution. In a control group injected intraperitoneally with HR-gp100, levels of anti-gp100 antibodies were 3 to 6 times higher than those in topically-treated mice (Figure 2).
Figure 2.
Serum anti-HR-gp100 antibody levels in mice treated topically with HR-gp100 or HR-gp100H. One group was treated intra-peritoneally with HR-gp100 (positive control), and one group with PBS (negative control). Results are from one representative experiment out of three performed. Each group included 6 mice. Three serum dilutions (1:200, 1:500, 1:1000) are shown. Bars indicate standard deviation of the mean.
3.2 Activation of Langerhans’ cells following topical HR-gp100 treatment of intact human skin
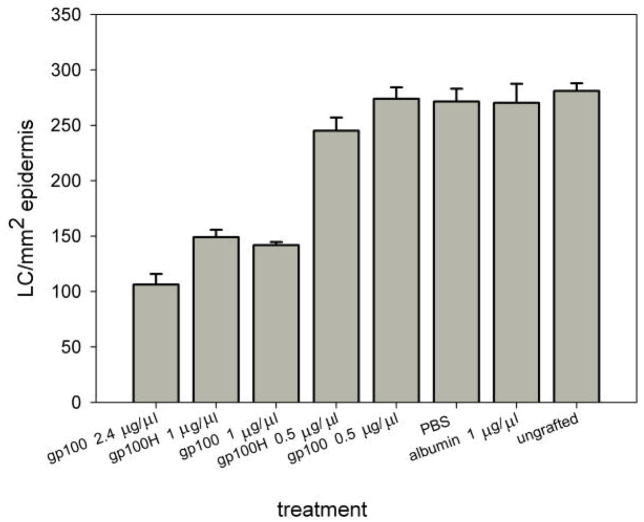
For the direct evaluation of LC activation and migration out of the epidermis following HR-gp100 treatment, we grafted human skin on the chicken chorioallantoic membrane. Each experiment included as negative controls at least two of the following: ungrafted human skin, skin incubated with solvent (Fig. 3A), and skin incubated with a control protein (bovine serum albumin, BSA). DNFB application to grafted skin was used as a positive control for LC activation [13] (Fig. 3B). After integration of the grafted skin with the CAM, the tested molecules were applied on the human skin; after overnight incubation, the skin was embedded in paraffin, sectioned and immunostained for CD1a (Figs 3C and 4). Quantitative evaluation of CD1a staining showed that HR-gp100 caused a significant decrease in epidermal LC number in a dose-dependent manner (2.4μg/ml vs. 1μg/ml p<0.016; 1μg/ml vs. 0.5μg/ml p<0.001; 0.5μg/ml vs. PBS not significant). Haptide- containing HR-gp100 (HR-gp100H) had a similar effect, and did not further increase LC migration (1μg/ml vs. 0.5μg/ml p<0.0009; 0.5μg/ml vs. PBS<0.05) (Fig. 5).
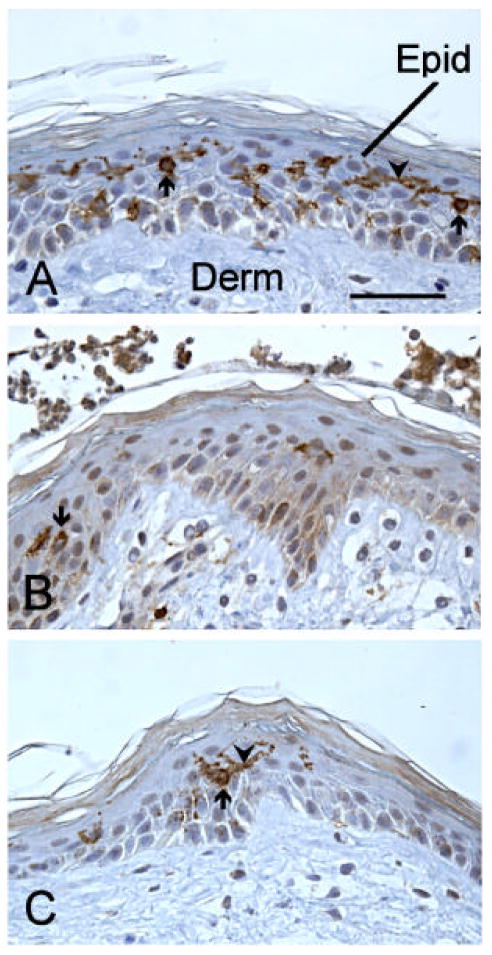
Figure 3.
Cross-sections of human skin grafted to the chick chorioallantoic membrane and stained with anti-CD1a to reveal LC. The sections are counterstained with hematoxylin to reveal the general structure of the skin. Panel A shows a control skin fragment treated overnight with solvent (PBS). A few of the many brown-stained LC are designated by arrows, and some of their processes are indicated by arrowheads. In B, a fragment treated with DNFB is shown. One LC, and a few stained processes can be seen. C shows a fragment treated overnight with nLT, with a single, large LC, with long process. Epid- epidermis, Derm- dermis, calibration bar = 50mm.
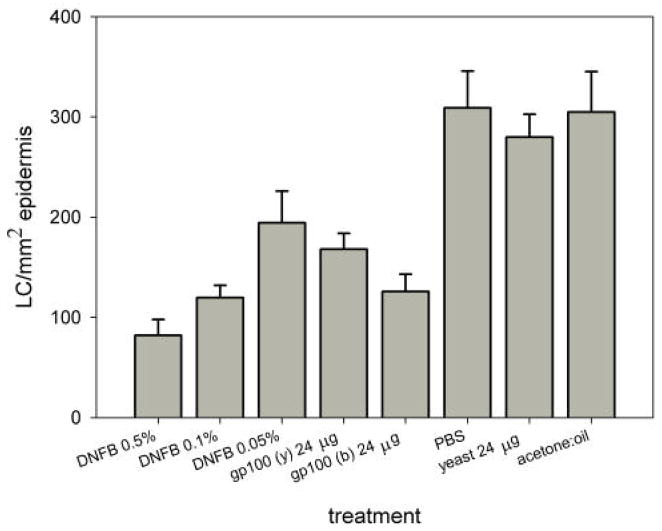
Figure 4.
Effect of topical application of HR-gp100 produced in yeast (y) or bacteria (b) on the number of LC in human epidermis. The tested molecules were applied overnight to human skin grafted to the chicken chorioallantoic membrane. Skin treated with DNFB served as a positive control. Skin treated with the solvent of HR-gp100 (PBS) or DNFB (acetone: olive oil ), and skin treated with the yeast carrying the wild type plasmid and processed in the same way as HR-gp100 (yeast)[10], served as negative controls. The number of LC cells normalized to the cross-sectional area of the epidermis examined is shown. The results of one of two experiments that yielded similar results are presented. Bars represent standard error of the mean.
Figure 5.
Effect of topical application of HR-gp100 and HR-gp100H on the number of LC in human epidermis. The tested molecules were applied overnight to human skin grafted to the chicken chorioallantoic membrane Ungrafted skin, skin treated with solvent (PBS) and skin treated with albumin served as negative controls The number of LC cells normalized to the cross-sectional area of the epidermis examined is shown. The results one of two experiment that yielded similar results are presented. Bars represent the standard error of the mean.
The effect on human DC migration induced by HR-gp100 produced in bacteria was also compared to the effect of HR-gp100 produced in yeast, and found to be similar (Fig. 3). These results are in contrast to the effects of these molecules on murine antibody production, which was found to be different for each substance (Fig. 1).
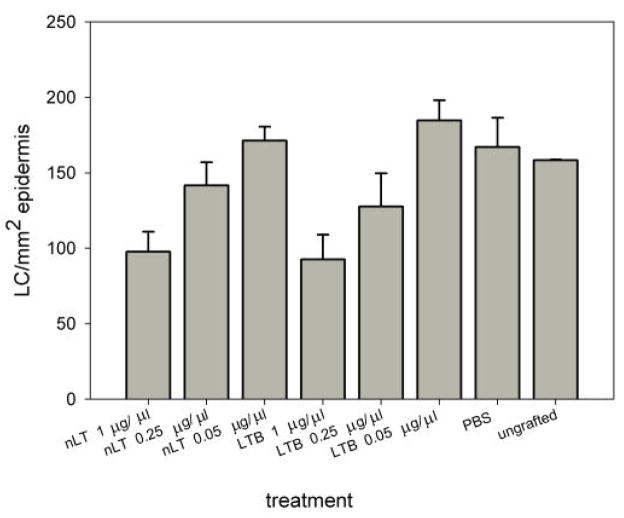
We also compared the number of CD1a-positive LC in the epidermis after the treatment of human skin with either nLT or LTB, since it is still an unresolved issue whether nLT or LTB is more efficient in stimulating dendritic cells. The results depicted in Fig. 6 clearly demonstrate that both molecules had a similar effect on LC migration.
Figure 6.
Comparison of the effect of topical application of nLT and LTB on the number of LC in human epidermis. The tested molecules were applied overnight on human skin grafted on the chicken chorioallantoic membrane. Non-grafted skin and skin treated with PBS served as negative controls. The number of LC cells normalized to the cross-sectional area of the epidermis examined is shown. The results one of two experiment that yielded similar results are presented. Bars represent the standard error of the mean.
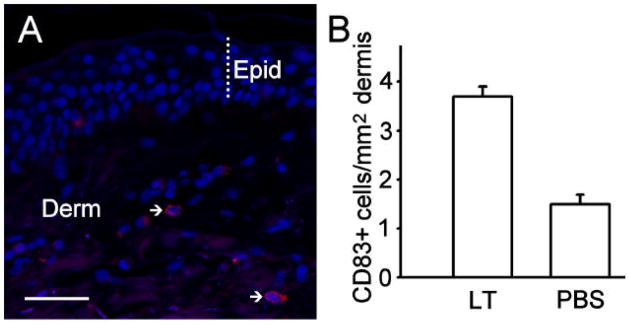
In order to determine that DC were activated, and to rule out the possibility that the decrease in CD1a+ cells in the epidermis was due to a low level of the marker on the cell membrane or cell death rather than to cell migration, DNFB- and nLT-treated skin was stained with a CD83 antibody. No CD83+ cells were observed with overnight incubation of the allergens. This is consistent with the known rapid activation and migration of LC in response to allergens. Therefore, we performed experiments where the skin was harvested at earlier time points and stained with CD83. Examination of sections from skin taken four hours after application of nLT revealed a significant increase in CD83+ cells in the upper dermis of DNFB-treated skin (Figs. 7A and B), compared to PBS-treated skin (p<0.001), but no difference in the epidermis. After 6 hours, the number of CD83+ cells in the dermis was already lower, and the difference with PBS-treated skin was not significant (not shown). CD1a stained cells were not observed in the dermis of treated skin after overnight incubation, and only rarely in untreated dermis or in dermis 4hours after treatment. These data support the conclusion that nLT induced activation, maturation, and migration of DC from the epidermis towards the dermis.
Figure 7.
Immunocytochemical localization of CD83 in the dermis of human skin grafted to the chick chorioallantoic membrane. A – A cross-section through a skin fragment that received an application of nLT four hours earlier. Arrows point to two activated LC cells expressing CD83+ (red staining) in the dermis (Derm). The thickness of the epidermis (Epid) is indicated by a dotted line. Nuclei are counterstained with Hoechst nuclear dye (blue). Calibration bar = 50mm. B- The skin treated with nLT or PBS is shown. A total of 144 cells in LT-treated skin and 83 cells in PBS-treated skin were counted. Bars represent the standard error of the mean.
CAM grafting maintains the integrity of human skin better than organ culture. However, in order to confirm ours results with the less in vivo-like but more widely used organ culture system, we performed two experiments testing the effects of nLT on intact human skin cultured at the air-liquid interface. As was the case with the CAM grafted skin, nLT produced a 60–70% migration of LC out of the epidermis, compared to PBS/albumin treated controls (22 and 81 cells/mm2 epidermis with nLT, compared to 81 and 289 cells/mm2 with PBS respectively).
4. Discussion
Our group, as well as others [3–8], has shown that both nLT and LTB are efficient carriers for transcutaneous antigen delivery. However, the issue of whether there is a requirement for antigen coupling to the bacterial enterotoxin carrier in order to attain efficacy has not been resolved and published results have not been consistent. In some cases, induction of cytotoxic T cell responses was effective after co-administration of enterotoxin-based adjuvants and antigen [21–23] or virus [24]. In other cases, gene fusion or chemical conjugation were necessary to elicit a strong immune response [25,26]. In our study, nLT or LTB co-administered with HR-gp100 did not further increase the response; in addition, when the molecules were co-administered, the response to HR-gp100 was somehow reduced. This could be due to the fact that antibodies were produced simultaneously against nLT (anti-CT [8]) and HR-gp100. It is possible that enhanced responses would have been obtained with a fused molecule. In this context, we found that fusion of HR-gp100 with the Haptide induced an increase in antibody production after skin application (Fig. 2). This indicates that Haptide sequences not only enhance transfection into cells [11,12], but can also promote trans-penetration through tissue layers. However, the fact that HR-gp100 seemed to interact with the membrane and penetrate cells on its own, may in this particular case make the carrier molecule less relevant.
We showed here that HR-gp100 produced in bacteria and in yeast, when delivered through the skin, induced a similar level of LC migration, ruling out the possibility of endotoxin-related activation. However, the two molecules induced different immune response patterns. Whereas HR-gp100 produced in yeast failed to induce antibody production, in vitro production of interferon-γ by spleen cells in response to the proteins was similar (results not shown). Although we do not have an explanation for this finding, it may be related to the recently observed complexity and heterogeneity of cutaneous dendritic cells subtypes and functions. For example, it was reported that virus delivered to the skin infected LC, but the cytotoxic response that developed was mediated by lymph node-derived CD8α+ DC and not by skin-derived LC [27]. Similarly, in a cutaneous murine leishmaniasis model, CD8α− and Langerin-negative DC, but not LC, acted as the principal antigen presenting cells [28]. These findings are in contrast to the previously accepted view that tissue resident LC acquire antigen in the peripheral compartments, and then migrate to draining lymph nodes to directly prime T cells. Thus, it is possible that differences between the yeast- and bacteria-produced protein, such as the degree of post translation glycosylation, induce different patterns of antigen presentation. This issue requires further clarification.
Transplantation of mammalian tissues to the pre-immune avian chorioallantoic membrane (CAM) is a widely used model for the study of many biological processes. Host blood vessels enter the transplanted tissues from the CAM and fuse with those in the graft to supply oxygen and nutrients essential for survival. This yields a low-cost system with characteristics that are close to normal tissue. In the current study, we have used for the first time the CAM system for delivering antigens to human skin, and measuring the resulting activation of LC. Using this system, we showed that transcutaneous delivery of HR-gp100 and nLT was followed by a dose-dependent decrease of epidermal LC number, and an increase in mature (CD83+) dendritic cells in the upper dermis. These data are consistent with the accepted view that LC display migratory properties that endow them with the unique capacity to journey between skin and draining lymph nodes where they encounter antigen-specific T lymphocytes. In addition, they support the conclusion that the allergens induced activation, maturation, and migration of DC from the epidermis towards the dermis in the treated skin.
Although the ability of the chicken CAM to support the survival of human skin has been shown in the past [16,17], to the best of our knowledge the current study is the first experimental application of this system.
Addition of nLT or LTB to HR-gp100 did not further affect the number of LC remaining in the epidermis. Results obtained from human skin using the CAM system, and the in vivo experiments with mice (see also [8]), show that nLT and LTB have similar effects on LC maturation, activation and antibody production.
Since nLT contains both the mutated A and the B subunits, this is likely to indicate that the effects observed take place through the homopentameric B subunit. This subunit possesses the capacity to enter mammalian cells and to activate cell-signaling events in leukocytes that modulate immune cell function [29,30].
In summary, we demonstrate here that an antigenic tumor-derived protein is delivered through the skin, activates Langerhans cells, and induces specific antibody responses. The full potential of this approach for immunostimulation deserves further research.
Acknowledgments
This work was supported by the Chief Scientist of the Israel Ministry of Industry and Commerce, and by NIH grant 1 R21 CA114160-01A1 (to S.F.). We wish to thank Dr. Gerard Marx from Hapto Biotech for his help and advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hammond SA, Walwender D, Alving CR, Glenn GM. Transcutaneous immunization: T cell responses and boosting of existing immunity. Vaccine. 2001;19:2701–7. doi: 10.1016/s0264-410x(00)00506-5. [DOI] [PubMed] [Google Scholar]
- 2.Cerundolo V, Hermans IF, Salio M. Dendritic cells: a journey from laboratory to clinic. Nat Immunol. 2004;5:7–10. doi: 10.1038/ni0104-7. [DOI] [PubMed] [Google Scholar]
- 3.Kotloff KL, Sztein MB, Wasserman SS, Losonsky GA, DiLorenzo SC, Walker RI. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect Immun. 2001;69:3581–90. doi: 10.1128/IAI.69.6.3581-3590.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neidleman JA, Vajdy M, Ugozzoli M, Ott G, O’Hagan D. Genetically detoxified mutants of heat-labile enterotoxin from Escherichia coli are effective adjuvants for induction of cytotoxic T-cell responses against HIV-1 gag-p55. Immunology. 2000;101:154–60. doi: 10.1046/j.1365-2567.2000.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simmons CP, Hussell T, Sparer T, Walzl G, Openshaw P, Dougan G. Mucosal delivery of a respiratory syncytial virus CTL peptide with enterotoxin-based adjuvants elicits protective, immunopathogenic, and immunoregulatory antiviral CD8+ T cell responses. J Immunol. 200;166:1106–13. doi: 10.4049/jimmunol.166.2.1106. [DOI] [PubMed] [Google Scholar]
- 6.Scharton-Kersten T, Glenn GM, Vassell R, Yu J, Walwender D, Alving CR. Principles of transcutaneous immunization using cholera toxin as an adjuvant. Vaccine. 1999;17 (Suppl 2):S37–43. doi: 10.1016/s0264-410x(99)00233-9. [DOI] [PubMed] [Google Scholar]
- 7.Beignon AS, Briand JP, Rappuoli R, Muller S, Partidos CD. The LTR72 mutant of heat-labile enterotoxin of Escherichia coli enhances the ability of peptide antigens to elicit CD4(+) T cells and secrete gamma interferon after coapplication onto bare skin. Infect Immun. 2002;70:3012–9. doi: 10.1128/IAI.70.6.3012-3019.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitcovski J, Bazak Z, Wasserman E, Elias O, Levy A, Peretz P, et al. Heat labile enterotoxin of E. coli – a potential adjuvant for Transcutaneous Cancer Immunotherapy. Vaccine. 2006;24:636–43. doi: 10.1016/j.vaccine.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 9.Gelbart Y, Frankenburg S, Pinchasov Y, Krispel S, Eliahu D, Drize O, et al. Production and purification of melanoma gp100 antigen and polyclonal antibodies. Protein Expression and Purification. 2004;34:183–189. doi: 10.1016/j.pep.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Frankenburg S, Elias E, Gelbart Y, Drize O, Lotem M, Ingber A, et al. Recombinant hydrophilic human gp100: uptake by dendritic cells and stimulation of autologous CD8+ lymphocytes from melanoma patients. Immunol Let. 2004;94:253–9. doi: 10.1016/j.imlet.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Gorodetsky R, Vexler A, Shamir M, An J, Levdansky L, Shimeliovich I, Marx G. New cell attachment peptide sequences from conserved epitopes in the carboxy termini of fibrinogen. Exp Cell Res. 2003;287:116–29. doi: 10.1016/s0014-4827(03)00120-4. [DOI] [PubMed] [Google Scholar]
- 12.Gorodetsky R, Levdansky L, Vexler A, Shimeliovich I, Kassis I, Ben-Moshe M, et al. Liposome transduction into cells enhanced by haptotactic peptides (Haptides) homologous to fibrinogen C-chain termini. J Controlled Release. 2004;95:477–488. doi: 10.1016/j.jconrel.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Pistoor FHM, Rambukkana A, Kroezen M, Lepoittevin JP, Bos JD, Kapsenberg ML, et al. Novel predictive assay for contact allergens using human skin explant cultures. Am J Pathol. 1996;149:337–343. [PMC free article] [PubMed] [Google Scholar]
- 14.Flaxman BA, Harper RA. Organ culture of human skin in chemically defined medium. J Invest Dermatol. 1975;64:96–99. doi: 10.1111/1523-1747.ep12510310. [DOI] [PubMed] [Google Scholar]
- 15.Varani J, Fligiel SEG, Perone P, Inman DR, Voorhees JJ. Effects of sodium lauryl sulfate on human skin in organ culture: comparison with all trans retinoic acid and epidermal growth factor. Dermatol. 1993:19–25. doi: 10.1159/000247191. [DOI] [PubMed] [Google Scholar]
- 16.Goodpasture W, et al. A study of human skin grafted to the chorio-allantois of chick embryos. J Exp Med. 1938;68:891–904. doi: 10.1084/jem.68.6.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunzi-Rapp K, Ruck A, Kaufmann R. Characterization of the chick chorioallantoic membrane model as a short-term in vivo system for human skin. Arch Dermatol Res. 1999;291:290–295. doi: 10.1007/s004030050410. [DOI] [PubMed] [Google Scholar]
- 18.Fingerut E, Gutter B, Meir R, Eliahoo D, Pitcovski J. Vaccine and adjuvant activity of recombinant subunit B of E. coli enterotoxin produced in yeast. Vaccine. 2005;23:4685–96. doi: 10.1016/j.vaccine.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 19.Tammi R, Jansen CT, Santti R. Histometric analysis of human skin in organ culture. J Invest Dermatol. 1979 Aug;73(2):138–40. doi: 10.1111/1523-1747.ep12581579. [DOI] [PubMed] [Google Scholar]
- 20.Romani N, Holzmann S, Tripp CH, Koch F, Stoitzner P. Langerhans cells - dendritic cells of the epidermis. APMIS. 2003;111:725–40. doi: 10.1034/j.1600-0463.2003.11107805.x. [DOI] [PubMed] [Google Scholar]
- 21.Simmons CP, Mastroeni P, Fowler R, Ghaem-maghami M, Lycke N, Pizza M, et al. MHC class I-restricted cytotoxic lymphocyte responses induced by enterotoxin-based mucosal adjuvants. J Immunol. 1999;163:6502–10. [PubMed] [Google Scholar]
- 22.Godefroy S, Goestch L, Plotnicky-Gilquin H, Nguyen TN, Schmitt D, Staquet MJ, et al. Immunization onto shaved skin with a bacterial enterotoxin adjuvant protects mice against respiratory syncytial virus (RSV) Vaccine. 2003;2:1665–71. doi: 10.1016/s0264-410x(02)00733-8. [DOI] [PubMed] [Google Scholar]
- 23.Kahlon R, Hu Y, Orteu CH, Kifayet A, Trudeau JD, Tan R, et al. Optimization of epicutaneous immunization for the induction of CTL. Vaccine. 2003;21:2890–9. doi: 10.1016/s0264-410x(03)00141-5. [DOI] [PubMed] [Google Scholar]
- 24.El-Ghorr AA, Williams RM, Heap C, Norval M. Transcutaneous immunisation with herpes simplex virus stimulates immunity in mice. FEMS Immunol Med Microbiol. 2000;29:255–61. doi: 10.1111/j.1574-695X.2000.tb01531.x. [DOI] [PubMed] [Google Scholar]
- 25.George-Chandy A, Eriksson K, Lebens M, Nordstrom I, Schon E, Holmgren J. Cholera toxin B subunit as a carrier molecule promotes antigen presentation and increases CD40 and CD86 expression on antigen-presenting cells. Infect Immun. 200;69:5716–25. doi: 10.1128/IAI.69.9.5716-5725.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haicheur N, Bismuth E, Bosset S, Adotevi O, Warnier G, Lacabanne V, et al. The B subunit of Shiga toxin fused to a tumor antigen elicits CTL and targets dendritic cells to allow MHC class I-restricted presentation of peptides derived from exogenous antigens. J Immunol. 2000;165:3301–8. doi: 10.4049/jimmunol.165.6.3301. [DOI] [PubMed] [Google Scholar]
- 27.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, et al. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1856–7. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 28.Ritter U, Meissner A, Scheidig C, Korner H. CD8 alpha- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur J Immunol. 2004;4:1542–50. doi: 10.1002/eji.200324586. [DOI] [PubMed] [Google Scholar]
- 29.Dickinson BL, Clements JD. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–23. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams NA. Immune modulation by the cholera-like enterotoxin B-subunits. Int J Med Microbiol. 2000;290:447–53. doi: 10.1016/S1438-4221(00)80062-4. [DOI] [PubMed] [Google Scholar]