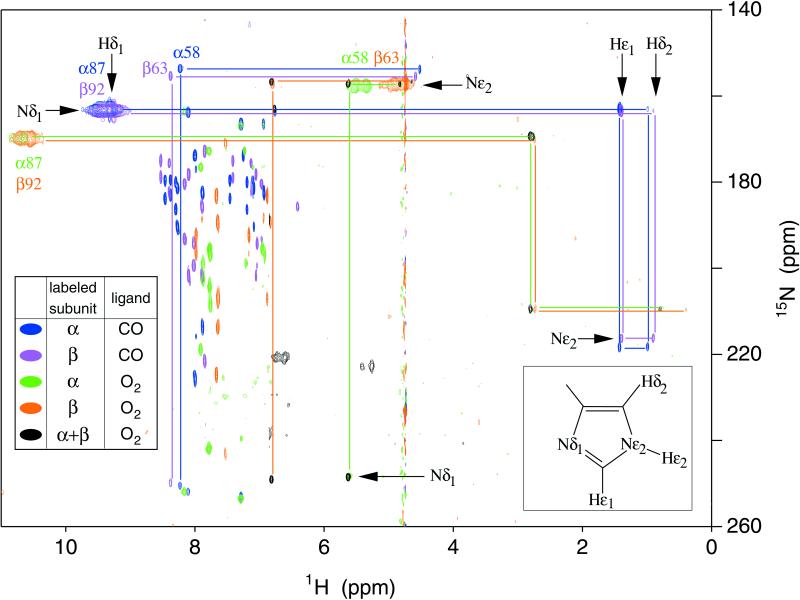
Figure 1.
Histidine region of the 600-MHz (1H, 15N) echo anti-echo HMQC spectra of chain-selectively and fully 15N-labeled HbCO A and HbO2 A in water and D2O at 29°C. Cross-peaks in blue, violet, green, and orange come from spectra of (15)N-α-labeled HbCO A, (15)N-β-labeled HbCO A, (15)N-α-labeled HbO2 A, and (15)N-β-labeled HbO2 A, respectively. These experiments used a large spectral width in 15N and a short refocusing delay (see Materials and Methods for details). The spectra were acquired without 15N decoupling; therefore doublets are observed for directly bonded (15)N-1H pairs. Also shown in green is a spectrum of (15)N-α-labeled HbO2 A with a smaller 15N spectral width and a longer refocusing delay, set to eliminate the α58His (Hɛ2, Nɛ2) doublet, which overlaps the Hɛ1 cross-peak at 5.64 ppm. Cross-peaks in black are those of fully 15N-labeled HbO2 A in D2O solution, which used a narrow 15N spectral width and a short refocusing delay. Cross-peaks originating from the same residue are connected by lines. Other cross-peaks originate from the solvent-exposed and interfacial histidyl residues not discussed in this paper (see ref. 17 for details).