Abstract
Focal accumulations of mononuclear cells in the arterial wall of healthy humans at predilection sites for atherosclerotic lesions have been described as ‘vascular-associated lymphoid tissue’ (VALT). Here we investigated whether pigs (Sus scrofa), a commonly used animal model for studying cardiovascular disease, have VALT. Samples of major arteries were collected from 10 conventional crossbred pigs (age, 2 to 24 mo) and processed for routine light microscopy, immunohistochemistry, and immunofluorescence. Single or small aggregates of mononuclear cells were noted in the intima and occasionally the inner portion of the tunica media and adventitia at branching sites. The infiltrating cells were primarily CD3+CD4+ T cells, with some macrophages. No CD8+ T cells were present. Infiltrating leukocytes and overlying endothelial cells frequently expressed major histocompatibility class II molecules. Two Ossabaw pigs on low-fat diet had similar leukocytic aggregates at locations where animals of the same breed but fed a high-fat and high-cholesterol diet developed atherosclerotic lesions. Further, the densities of CD3+ T lymphocytes and in these areas were decreased in 2 sedentary and 2 exercised Ossabaw pigs on an atherogenic diet compared with conventional crossbred and Ossabaw pigs on a normal diet. This study shows that focal aggregates of lymphocytes occur in the vasculature of pigs at locations predisposed to development of atherosclerotic lesions. These cellular aggregates are similar to the structures described as VALT in human arteries and reinforce the value of the pig as a model for the study of human cardiovascular disease.
Abbreviations: MAC387, myeloid–histiocytic antigen; MNC, mononuclear cells; TCR, T cell receptor; VALT, vascular-associated lymphoid tissue
The pathogenesis of atherosclerosis involves immune and inflammatory mechanisms.18 Atherosclerotic lesions develop in the arterial vasculature in areas of high hemodynamic stress,11,19 but the reasons for this predilection are not well understood. In children and adolescents, considerable numbers of mononuclear cells in the vascular wall accumulate in areas predisposed for development of atherosclerotic lesions later in life.11,18 These cellular accumulations, termed ‘vascular-associated lymphoid tissue’ (VALT) in analogy to mucosa-associated lymphoid tissue, are assumed to function as a local immunosurveillance system.11,18
Pigs frequently are used as an animal model for the study of cardiovascular disease. The pig offers advantages in terms of size, anatomy, physiology, and endocrine and cardiovascular disease development, which are more similar to those of humans than are those of mice and rabbits,17 in which most studies of atherosclerosis have been performed. Whereas an abundance of dendritic cells and macrophages were noted in the arterial intima of atherosclerosis-predisposed regions in the aorta of wild-type mice7 and normocholesterolemic rabbits,10 only a few intimal lymphocytes were seen at these sites in these 2 species. The present study demonstrates the presence of mononuclear cell aggregates with abundant lymphocytes analogous to VALT in humans at the branching sites of major arteries in conventional crossbred and Ossabaw pigs, a novel model of the metabolic syndrome in humans that is uniquely suited for the study of cardiovascular disease.4
Materials and Methods
Tissue specimens.
Samples of major branching sites of thoracic and abdominal aorta and common carotid artery, left anterior descending and right coronary arteries, and brachial and femoral arteries were collected from 10 conventional crossbred pigs submitted for necropsy to the Indiana Animal Disease Diagnostic Laboratory (West Lafayette, IN). The specific anatomic locations were the origin of the brachiocephalic trunk and left subclavian artery for the thoracic aorta; the origin of the external and internal iliac arteries for the caudal aorta; the branching site of the external and internal carotid arteries for the common carotid artery; the origin of the proximal and distal collateral branches located at the midportion of the left anterior descending coronary artery; the origin of the interventricular branches for the right coronary artery; the origin of the muscular branch for the brachial artery; and the origin of the lateral circumflex femoral artery for the femoral artery. The animals came from a small (approximately 240 sows) commercial breeding farm in Indiana and were culled for various health concerns (Table 1). They varied in age from 2 mo to approximately 2 y, with an average of 5.3 mo. Most (90%) of the animals were younger than 4.5 mo old. Animals were first chemically restrained by using succinyl choline then rendered unconscious by electrical stunning, and finally euthanized by electrocution.1 In addition, similar samples at branching sites of major arteries were collected from six 2-y-old, male Ossabaw pigs divided into 2 diet groups: (1) 3 lean pigs fed a normal maintenance diet (7% kcal from fat), and (2) 3 obese pigs fed a high fat diet (45% kcal from fat and 2% cholesterol) for 57 wk; 2 of the latter animals underwent aerobic exercise for 5 wk. The Ossabaw pigs originated from a breeding colony maintained by the Indiana University School of Medicine and Purdue University. The experimental procedures involving these animals, including euthanasia by pneumothorax and cardiectomy while anesthesized with isoflurane gas, were approved by the Institutional Animal Care and Use Committee of Indiana University and fully complied with the Guide for the Care and Use of Laboratory Animals.12
Table 1.
Vascular-associated lymphoid tissue at the branching sites of the abdominal aorta and the carotid artery in 10 conventional crossbred pigs
| Abdominal aorta |
Carotid artery |
|||||
| Pig | Age (mo) | Reason for culling | CD3 | MAC387a | CD3 | MAC387 |
| 1 | 4 | Chronic polyserositis; bronchopneumonia | ++++ | ± | ND | ND |
| 2 | 3 | Megacolon associated with rectal stricture | +++ | + | ++ | ± |
| 3 | 3 | Nonunion fracture of left humerus | ND | ND | ± | – |
| 4 | 3 | Bronchopneumonia | +++ | – | ++ | – |
| 5 | 4 | Bronchopneumonia | +++ | ± | + | – |
| 6 | 4.5 | Chronic cellulitis and osteomyelitis of tail stump | +++ | + | ++ | ± |
| 7 | 2 | Umbilical hernia; rectal prolapse | ++ | ± | + | ± |
| 8 | 3 | Umbilical hernia; bronchopneumonia | ++ | ± | ++ | ± |
| 9 | 2.5 | Rectal prolapse; bronchopneumonia | +++ | – | + | – |
| 10 | 24 | Focal myelomalacia | +++ | + | + | ± |
–, immunohistochemistry (IHC)-negative; ±, average of less than 2 IHC-positive cells per 250 μm of intimal lining; +, average of 3 to 5 IHC-positive cells per 250 μm of intimal lining; ++, average of 6 to 9 IHC-positive cells per 250 μm of intimal lining; +++, average of 10 to 15 IHC-positive cells per 250 μm of intimal lining; ++++, average of more than 15 IHC positive cells per 250 μm of intimal lining; ND, not determined
myeloid-histiocytic antigen.
Histology and immunohistochemistry.
The tissue samples were fixed in formalin and processed for routine light microscopy and immunohistochemistry according to a standardized methodology.14 Briefly, the standard immunohistochemical procedure consisted of deparaffination and hydration of slides with 5-μm-thick serial sections; pretreatment with steam (for CD3) or proteinase K (for myeloid–histiocytic antigen); blocking of endogenous peroxidase with 0.3% H2O2; blocking of nonspecific binding; and treatment with primary antibody (rabbit antiCD3 [Dako, Carpintaria, CA] or mouse antimyeloid–histiocytic antigen MAC387 [Dako]) or negative control reagent; and subsequent treatment with biotinylated antirabbit antibody, streptavidin–peroxidase conjugate, and the chromogen diaminobenzidine (LSAB+ Visualization System, Dako] or with peroxidase-labeled polymer conjugated to goat antimouse antibody and the chromogen 3-amino-9-ethylcarbazole (Envision+ System, Dako), respectively. Slides were counterstained with Mayer hematoxylin, and sections were examined by fluorescence microscopy (Eclipse E400, Nikon, Melville, NY). Sections of lymph node used as positive controls for the immunohistochemical protocol were stained in conjunction with the vascular samples. Lymph node samples showed appropriate localization of positive cells, namely CD3+ T cells in the paracortex, and MAC387+ cells in the medulla and scattered throughout the paracortex. A semiquantitative analysis of the frequency of intimal mononuclear cells in the sampled arteries was undertaken at 400× magnification. The intimal lining of the entire luminal circumference of the vessel was measured in 3 cross-sections per sampling site. Stained cells in each field were counted, and the average number of cells per 250 μm of intimal lining was calculated.
Immunofluorescence.
Tissue samples were collected from arteries at the same locations as those for formalin fixation and were snap-frozen in liquid nitrogen for immunofluorescence studies with antibodies against CD4 (74-12-4),13 CD8 (76-2-11),13 and major histocompatibility complex class II antigen (MSA3).5 In summary, 8-μm-thick frozen sections on glass slides were air-dried at room temperature and blocked for nonspecific antibody binding with PBS containing 1% bovine serum albumin and 10% rabbit serum for 15 min. Sections were rinsed once in PBS and incubated for 30 to 60 min with the primary monoclonal antibody. Negative control sections received 1:10 dilution of PBS containing 1% bovine serum albumin and 10% rabbit serum. Incubation took place in a humidified chamber at room temperature. The sections were then rinsed 3 times in PBS, the 1:100-diluted fluorescein isothiocyanate-conjugated secondary antibody (goat antimouse IgG, Jackson ImmunoResearch, West Grove, PA) was applied, and the sections were incubated for another 30 min. After 3 further rinses in PBS, sections were covered with 4’,6-diamidino-2-phenylindole (5 μg/ml; Boehringer Mannheim)–methanol and incubated for 15 min at room temperature. After washing once with methanol, slides were mounted in antifading mounting medium. Sections were examined by fluorescence microscopy (Eclipse E400, Nikon) at 400× magnification. Lymph node was used as the positive control tissue.
Results
Gross findings.
The arteries examined from the conventional crossbred and the lean Ossabaw pigs were unremarkable grossly. All obese Ossabaw pigs had firm, raised, white or yellow plaques with rare calcified foci causing intimal thickening of the abdominal aorta at the bifurcation site of the iliac arteries.
Microscopic findings.
(i) Conventional crossbred pigs.
Single or small aggregates of mononuclear cells (MNC) were noted in the vessel wall of the aorta and carotid artery from the conventional pigs, predominantly in the intima (Figure 1) but occasionally also within the inner portion of the tunica media and around the vasa vasorum in the adventitia. The infiltrating cells were predominantly cells that reacted with the antiCD3 antibody specific for T cells (Figures 2 and 3), with most of these cells coexpressing CD4, a marker for T helper cells. Other cells were identified as macrophages based on positive reaction with MAC387 and characteristic nuclear morphology. The antiCD8 antibody, which recognizes cytotoxic T cells, did not react with any cells. Infiltrating leukocytes and overlying endothelial cells frequently expressed major histocompatibility complex class II molecules (Figure 4). Endothelial cells were positive for class II molecules where activated leukocytes occurred in the vicinity.
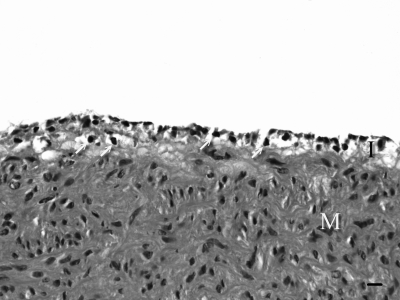
Figure 1.
Carotid artery, 4-mo-old conventional pig. Multiple individual mononuclear leukocytes (arrows) expand the intima (I). M, tunica media. Hematoxylin and eosin stain. Bar, 10 μm.
Figure 2.
Abdominal aorta, 4-mo-old conventional pig. Mononuclear leukocytes are located primarily in the intima and inner media. Labeled streptavidin–biotin–peroxidase immunohistochemistry for CD3, with Mayer hematoxylin counterstain. Bar, 100 μm.
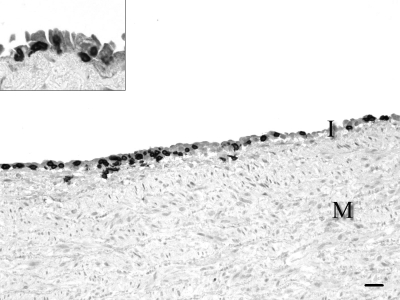
Figure 3.
Abdominal aorta, 2.5-mo-old conventional pig. The mononuclear cells in the intima (I) are predominantly T lymphocytes closely associated with the endothelium (inset, higher magnification). M, tunica media. Labeled streptavidin–biotin–peroxidase immunohistochemistry for CD3, with Mayer hematoxylin counterstain. Bar, 25 μm.
Figure 4.
Abdominal aorta, 2-mo-old conventional pig. Numerous cells (arrows) along the intimal lining (I) are positive for MHC-II. L, vascular lumen; M, tunica media. Immunofluorescence for major histocompatibility class II antigen. Bar, 10 μm.
MNC were concentrated in the wall opposite the flow-dividing septum of the bifurcation sites and were most abundant at the branching site of the abdominal aorta into the iliac arteries (Table 1). In vessels with intimal thickening, MNC accumulated underneath the plaques (data not shown). Younger pigs were as likely to have VALT as was the adult (2-y-old) pig (Table 1). MNC in regions further proximal or distal to the branches were very rare. The other arteries examined (left anterior descending and right coronary, brachial, and femoral), as well as the veins and lymphatics present in some of the sections, did not have any leukocytic infiltration.
(ii) Ossabaw pigs.
In the current study, accumulations of T lymphocytes and macrophages similar to the cells noted in the conventional pigs were present in the lean Ossabaw pigs at locations where atherosclerotic lesions form when these animals are fed a high-fat, high-cholesterol diet, namely, at the bifurcation of the abdominal aorta and common carotid artery. The atheromas were characterized by lipid cores with cholesterol clefts, necrosis, and small foci of calcification, variably surrounded by foam cells, fibrous connective tissue, and proliferating smooth muscle cells (Figure 5). The density (number of cells per 250 µm endothelial lining) of CD3+ T lymphocytes and macrophages was decreased in these areas (Table 2).
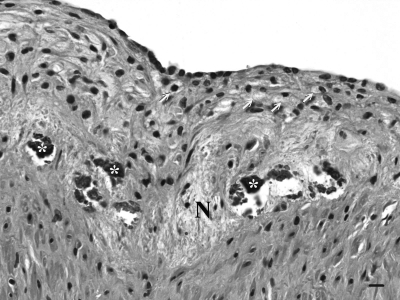
Figure 5.
Abdominal aorta, adult Ossabaw pig receiving a high-fat, high-cholesterol diet and aerobic exercise. In areas with atherosclerosis, the intima is markedly expanded by foam cells (arrows), fibrous connective tissue, smooth muscle cells, areas of necrosis (N), and small foci of calcification (*). Hematoxylin and eosin stain. Bar, 10 μm.
Table 2.
Vascular-associated lymphoid tissue at the branching sites of the abdominal aorta and the carotid artery in 6 Ossabaw pigs
| Abdominal aorta |
Carotid artery |
|||
| Pig (treatment) | CD3 | MAC387 | CD3 | MAC387 |
| 1 (obese) | + | + | – | – |
| 2 (obese) | + | ± | ± | – |
| 3 (obese + exercise) | + | + | ± | ± |
| 4 (obese + exercise) | + | ++ | + | – |
| 5 (lean) | ++ | ± | ± | – |
| 6 (lean) | +++ | +++ | ± | – |
–, immunohistochemistry (IHC)-negative; ±, average of less than 2 IHC-positive cells per 250 μm of intimal lining; +, average of 3 to 5 IHC-positive cells per 250 μm of intimal lining; ++, average of 6 to 9 IHC-positive cells per 250 μm of intimal lining; +++, average of 10 to 15 IHC-positive cells per 250 μm of intimal lining; ++++, average of more than 15 IHC positive cells per 250 μm of intimal lining
Histiocytic myeloid antigen
Discussion
The occurrence and composition of MNC aggregates within the vessel walls of these pigs is analogous to those forming the VALT in children and adolescents. Most (9 of 10) of the crossbred pigs in the present study were young (4.5 mo of age or younger). No marked differences were noted between the cell aggregates in these animals and those in the adult (2-y-old) crossbred animal and the Ossabaw pigs on a low-fat diet. The absence of age-related changes in the composition of the cellular aggregates in the vessel walls of these animals likely is related to the low-fat diet that the animals had received since weaning. Risk factors for atherosclerosis, such as hyperlipidemia, might interfere with the composition and immune function of the VALT. This concern is supported further by the visible reduction in the number of lymphocytes in the adult Ossabaw pigs on high-fat diet.
Some authors have suggested that MNC aggregates are always present in arterial regions at bifurcation sites subject to pronounced hemodynamic stress.11,18-20 Although the relative proportion of macrophages and T cells at these locations is variable, T cells clearly predominate in the aggregates in human arteries,11,18-20 as they did in the pigs we evaluated. Although 1 study investigating the involvement of VALT in immune responses in abdominal aortic aneurysms reported finding typical lymphoid follicles, with B cells forming germinal centers,2 the structure of VALT usually is not as highly organized as that of mucosa-associated lymphoid tissue. Instead, VALT is more similar in structure to bronchus-associated lymphoid tissue and the lymphoid tissue of the inner ear.18
In general, more CD4+ than CD8+ T cells are present in the VALT of humans,11,18-20 indicating a predominance of T cells regulating cell-mediated immune responses and antibody production rather than of T cells with cytotoxic activity. Reportedly most of these cells in the vessel walls express the α/β T cell receptor (TCR), but an unexpectedly high number were also positive for TCRγ/δ.11,18-20 Although the specific percentage of TCRγ/δ+ cells was not reported in the cited studies, the numbers were indicated to parallel previous observations of considerably large proportions of these cells in early atherosclerotic lesions.8 Nearly 10% of T cells in the transition zone between normal intima and fatty streaks carried TCRγ/δ, a proportion that reportedly decreased to 6.6% and 4.3% in fatty streaks and atherosclerotic plaques, respectively.8 In the present study, the T cells in the aggregates were mostly CD4+ lymphocytes; no CD8+ T cells were noted in the vessels of the pigs examined. The remaining T cells in the vascular wall may have been γ/δT cells that were CD2–CD4–CD8–, a substantial subpopulation of cells in the blood and lymphoid tissues of pigs, primarily in young animals.22
In addition to T cells and macrophages, dendritic cells and some mast cells were identified in the intima in the carotid branching area of children and adolescents,11,18 whereas NK cells, polymorphonuclear granulocytes, and B cells were virtually absent from the arteries examined.11,18,20 The lymphoid follicles in which B cells were forming germinal centers that were reported in association with abdominal aortic aneurysms2 do not appear to be a feature of VALT; they likely form in response to localized chronic inflammation in the affected region of the vessel wall.
In both humans18,20 and pigs, MNC were found not only in the intima but also around the vasa vasorum; however, the aggregates were more numerous in the inner vascular wall. Therefore, the theory that the MNC forming the VALT originate from the vascular lumen is favored over the supposition that they arrive via the vasa vasorum.18 These observations are analogous to findings from a study of vascular MNC in mice, in which the cellular composition and distribution in the intima of the aorta differed from those in the adventitia.7 Whereas leukocytes were abundant throughout the entire adventitia in several mouse strains, independently of whether they were atherosclerosis-resistant, there was a spatial and quantitative correlation between dendritic cells in distinct regions of the normal aortic intima, the topography of atherosclerotic lesion formation, and the genetic susceptibility of mice to atherogenesis.
MNC rarely, if ever, were present in the intima of veins.19,20 The veins and lymphatics in the pigs did not show any leukocytic cell infiltrate. In addition to other factors, such as increased blood pressure, this distribution may explain why lesions of atherosclerosis develop in arteries but not in veins or lymphatics.
The conventional pigs used in this study were not completely healthy, unlike the human subjects in which VALT initially was recognized.11,18 The animals had been culled for various reasons, including umbilical hernia, fractures, rectal prolapse, and bronchopneumonia; however, we took care during the selection of animals for this study to exclude those that had known concurrent compromise of the vascular system. We consider that the intimal MNC aggregates in our pigs are unrelated to their various disease processes. Nonetheless, the increased circulating levels of acute-phase proteins and cytokines during disease may have led to activation and recruitment of more leukocytes into areas with VALT than might have occurred in fully healthy animals. This difference in leukocyte recruitment rather than interindividual variation may have accounted for the higher number of lymphocytes in the intima of the abdominal aorta in 1 of our pigs, which had bronchopneumonia and chronic polyserositis, indicative of systemic bacterial disease.
Research on healthy arteries from 8-wk to 10-y-old children revealed VALT at sites prone to atherosclerosis.11,18 Mapping of sudanophilic lesions (corresponding to fatty streaks) in cholesterol-fed minipigs has demonstrated a similar topographic distribution in the aorta, iliac, and coronary arteries,3 specifically at arterial branching points and other vascular sites exposed to high mechanical (turbulence) stress. These sites also were affected by atherosclerosis in the obese Ossabaw pigs. Therefore, the distribution of the atherosclerotic lesions associated with the metabolic syndrome appears to correlate well with the occurrence of MNC in the vessel wall in both humans and pigs.
Normal intimal thickening due to cellular accumulation, so-called ‘intimal cell masses,’ can occur in the distal portion of the abdominal aorta and proximal portion of the coronary arteries in areas where atherosclerosis tends to arise in both swine on atherogenic diets and humans.9,16 Although these structures bear superficial resemblance to VALT, they are not considered to be equivalent, because the composition of the aggregates differs. Intimal cell masses consist predominantly (approximately 90%) of smooth muscle cells and extracellular matrix, such as collagen, elastic tissue, and glycosaminoglycans, with monocyte-like cells forming only a minor component (approximately 10%).16 In contrast, the prevailing cell population that forms the VALT are lymphocytes. One might speculate that intimal cell masses form at sites of VALT, but intimal cell masses are already well established at birth15 and therefore are regarded as constitutional elements of the vascular wall.
The possible immune and inflammatory mechanisms in atherogenesis have been the subject of ongoing debate. T lymphocytes are the first cells to appear in the intima of arteries predetermined for atherosclerotic lesions, followed by macrophages and smooth muscle cells.21 The cellular kinetics of the progression from fatty streak to atherosclerotic plaque reportedly undergo a characteristic change from predominantly T lymphocytic inflammatory infiltration, to a lymphocyte–macrophage stage, to a final stage where smooth muscle cells prevail.21 This progression of the lesion likely reflects the effect of activated T cells in the vascular wall, including indirect recruitment of macrophages through induction of expression of vascular cell adhesion molecule 1 and indirect stimulation of smooth muscle proliferation by means of interferon-induced macrophage activation.6 The prolonged life span and increased resistance to injury of smooth muscle cells15 may explain why they are the predominant cell population in well-developed atheromas. Oxidized low-density lipoproteins have been suggested as antigens that incite the cellular immune response that leads to atherosclerosis in humans; these low-density lipoproteins also further induce the expression of vascular cell adhesion molecule 1 in endothelial cells.6 The decrease in MNC and concurrent development of fibroatheromas in our pigs that received a high-fat, high-cholesterol diet suggest that a similar alteration takes place in pigs. These data corroborate the hypothesis that a cell-mediated immune response is the triggering mechanism of atherosclerosis.6
In summary, the T lymphocytes that form the VALT may play a role in the initiation and progression of atherosclerotic lesions. However, most studies of cardiovascular disease in humans have focused on late stages of atherosclerosis. This study further substantiates pigs as a suitable animal model for studying the early development of atherosclerosis.
Acknowledgments
This study was supported by NIH grants RR013223 and HL062552 and by the Purdue Comparative Medicine Center.
References
- 1.American Veterinary Medical Association. AVMA guidelines on euthanasia [Internet]. Schaumburg (IL): AVMA; June 2007 [cited 2007 Jul 14]. Available from: www.avma.org/resources/euthanasia.pdf. [Google Scholar]
- 2.Bobryshev YV, Lord RSA. 2001. Vascular-associated lymphoid tissue (VALT) involvement in aortic aneurysm. Atherosclerosis 154:15–21 [DOI] [PubMed] [Google Scholar]
- 3.Cornhill JF, Barrett WA, Herderick EE, Mahley RW, Fry DL. 1985. Topographic study of sudanophilic lesions in cholesterol-fed minipigs by image analysis. Arteriosclerosis 5:415–426 [DOI] [PubMed] [Google Scholar]
- 4.Dyson MC, Alloosh M, Vuchetich JP, Mokelke EA, Sturek M. 2006. Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comp Med 56:35–45 [PubMed] [Google Scholar]
- 5.Hammerberg C, Shurig GG. 1986. Characterization of monoclonal antibodies directed against swine leukocytes. Vet Immunol Immunopathol 11:107–121 [DOI] [PubMed] [Google Scholar]
- 6.Hansson GK, Libby P. 1996. The role of the lymphocytes. : Fuster V, Ross R, Topol EJ. Atherosclerosis and coronary artery disease. Philadelphia: Lippincott-Raven Publishers; p 557–568 [Google Scholar]
- 7.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. 2006. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med 203:2073–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleindienst R, Xu Q, Willeit J, Waldenberger FR, Weimann S, Wick G. 1993. Immunology of atherosclerosis. Demonstration of heat shock protein 60 expression and T lymphocytes bearing alpha/beta or gamma/delta receptor in human atherosclerotic lesions. Am J Pathol 142:1927–1937 [PMC free article] [PubMed] [Google Scholar]
- 9.Lee KT. 1986. Swine as animal models in cardiovascular research. Tumbleson ME. Swine in biomedical research. New York: Plenum Press; p 1481–1496 [Google Scholar]
- 10.Malinauskas RA, Herrmann RA, Truskey GA. 1995. The distribution of intimal white blood cells in the normal rabbit aorta. Atherosclerosis 115:147–163 [DOI] [PubMed] [Google Scholar]
- 11.Millonig G, Schwentner C, Mueller P, Mayerl C, Wick G. 2001. The vascular-associated lymphoid tissue: a new site of local immunity. Curr Opin Lipidol 12:547–553 [DOI] [PubMed] [Google Scholar]
- 12.National Research Council 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 13.Pescovitz MD, Lunney JK, Sachs DH. 1984. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol 133:368–375 [PubMed] [Google Scholar]
- 14.Ramos-Vara JA, Beissenherz ME. 2000. Optimization of immunohistochemical methods using two different antigen retrieval methods on formalin-fixed, paraffin-embedded tissues: experience with 63 markers. J Vet Diagn Invest 12:307–311 [DOI] [PubMed] [Google Scholar]
- 15.Stary HC. 1999. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis 9(1 Suppl):I19–I32 [PubMed] [Google Scholar]
- 16.Thomas WA, Lee KT, Kim DN. 1986. Swine as animal models in cardiovascular research. : Tumbleson ME. Swine in biomedical research. New York: Plenum Press; p. 1511–1525 [Google Scholar]
- 17.Tumbleson ME, Schook LB. 1996. Advances in swine in biomedical research. : Tumbleson ME, Schook LB. Advances in swine in biomedical research. New York: Plenum Press; p. 1–6 [Google Scholar]
- 18.Waltner-Romen M, Falkensammer G, Rabl W, Wick G. 1998. A previously unrecognized site of local accumulation of mononuclear cells: the vascular-associated lymphoid tissue. J Histochem Cytochem 46:1347–1350 [DOI] [PubMed] [Google Scholar]
- 19.Wick G, Knoflach M, Xu Q. 2004. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol 22:361–403 [DOI] [PubMed] [Google Scholar]
- 20.Wick G, Romen M, Amberger A, Metzler B, Mayr M, Falkensammer G, Xu Q. 1997. Atherosclerosis, autoimmunity, and vascular-associated lymphoid tissue. FASEB J 11:1199–1207 [DOI] [PubMed] [Google Scholar]
- 21.Xu QB, Oberhuber G, Gruschwitz M, Wick G. 1990. Immunology of atherosclerosis: cellular composition and major histocompatibility complex class II antigen expression in aortic intima, fatty streaks, and atherosclerotic plaques in young and aged human specimens. Clin Immunol Immunopathol 56:344–359 [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Parkhouse RM. 1996. Phenotypic classification of porcine lymphocyte subpopulations in blood and lymphoid tissues. Immunology 89:76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]