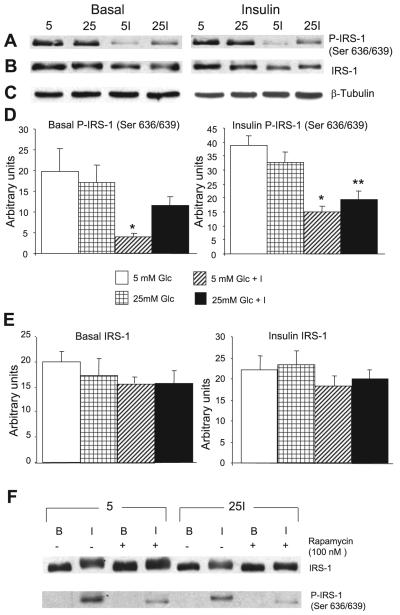
Fig. 6.
Effect of acute insulin stimulation on the phosphorylation of Ser636/639 of insulin receptor substrate (IRS)-1 is modified by the conditions of chronic preincubation and by rapamycin. Cells were processed as in Figs. 3 and 5 and then lysed and analyzed by Western blotting. A: representative Western blot prepared from cells that had been lysed in the basal state (left) or after 15 min of acute stimulation with 100 nM insulin (right) before lysis and immunoblotting with an antibody specific to P-IRS-1 hSer636/639. The conditions of preincubation were 5 mM glucose (5), 25 mM glucose (25), 5 mM glucose + 0.6 nM insulin (5I), and 25 mM glucose + 0.6 nM insulin (25I). Cells that were not stimulated acutely with insulin (basal) showed no evidence of phosphorylation when developed under the same conditions as the cells that had been acutely stimulated with insulin, as shown in F. The Western blot shown in A prepared from cells in the basal state was developed using WestDura ECL Reagent (Pierce) to increase sensitivity instead of the West Pico ECL reagent used routinely. B: identical to A except that the gel was stripped and developed with an antibody to total IRS-1. C: blots with β-tubulin antibody as a loading control. D and E: quantitative analyses of A and B, respectively. *P < 0.001 and **P < 0.005, all compared with control preincubated in 5 mM glucose; n = 4 for cells in the basal condition and n = 7 for cells after acute stimulation with insulin. F: cells that were preincubated in 5 mM glucose or in 25 mM glucose + 0.6 nM insulin and then either left in the basal state (B), or acutely stimulated with 100 nM insulin (I) for 15 min. Half of the dishes were exposed to 100 nM rapamycin for 30 min. Neither insulin nor rapamycin affected the total expression of IRS-1. Acute insulin without rapamycin slightly retarded the migration of IRS-1, and this was abolished by rapamycin. Acute insulin stimulated the phosphorylation of IRS-1 on Ser636/639, and this was diminished in cells that were preincubated in 25 mM glucose + low-dose insulin. Rapamycin markedly inhibited the phosphorylation of IRS-1. The gel is representative of 4 similar gels, from two separate experiments.