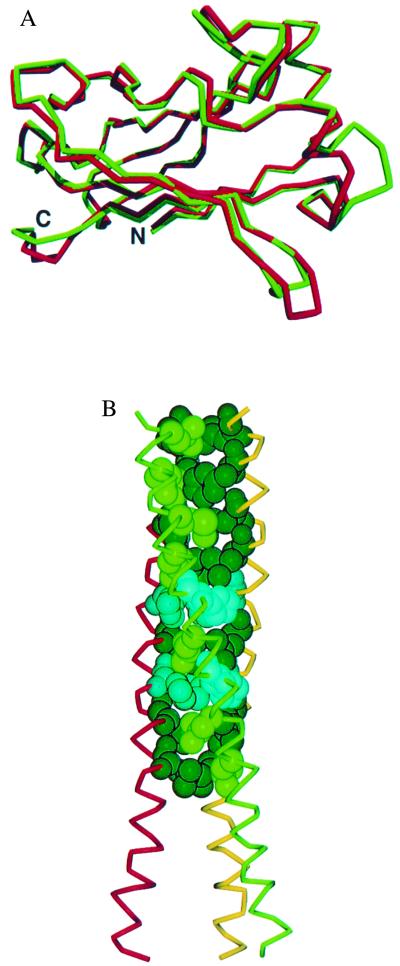
Figure 1.
The overall structure of the TRAF3 subunit is composed of an elongated helix followed by an eight-stranded β-sandwich (TRAF domain). (A) Superimposition of the polypeptide backbone of the C-terminal TRAF domain of TRAF3 (red) and TRAF2 (24). The rms deviation between corresponding α-carbons is 1 Å. Strong homology (59% identical) exists between TRAF3 and TRAF2 in this domain. The sandwich is formed by two layers of β-sheet, each with four antiparallel strands. The topology of this β-sandwich is found thus far only in TRAF domains. (B) The TRAF-N domain of TRAF3 is a long amphipathic α-helix that forms a coiled-coil when TRAF3 trimerizes (see Fig. 3). Residues 300–347 were ordered in the electron density map. The coiled-coil interactions are stabilized by nine heptad repeats of hydrophobic residues. This hydrophobic pattern is interrupted at residues 324 and 331 where three histidines are found in the interior of the coiled-coil. The side chains of these histidines extend out of the coiled-coil. The TRAF3 fragment is considerably longer at the N terminus than the TRAF2 fragment, where residues from the N terminus were missing or disordered (24, 33). This model provides structural details for most of the helical TRAF-N region.