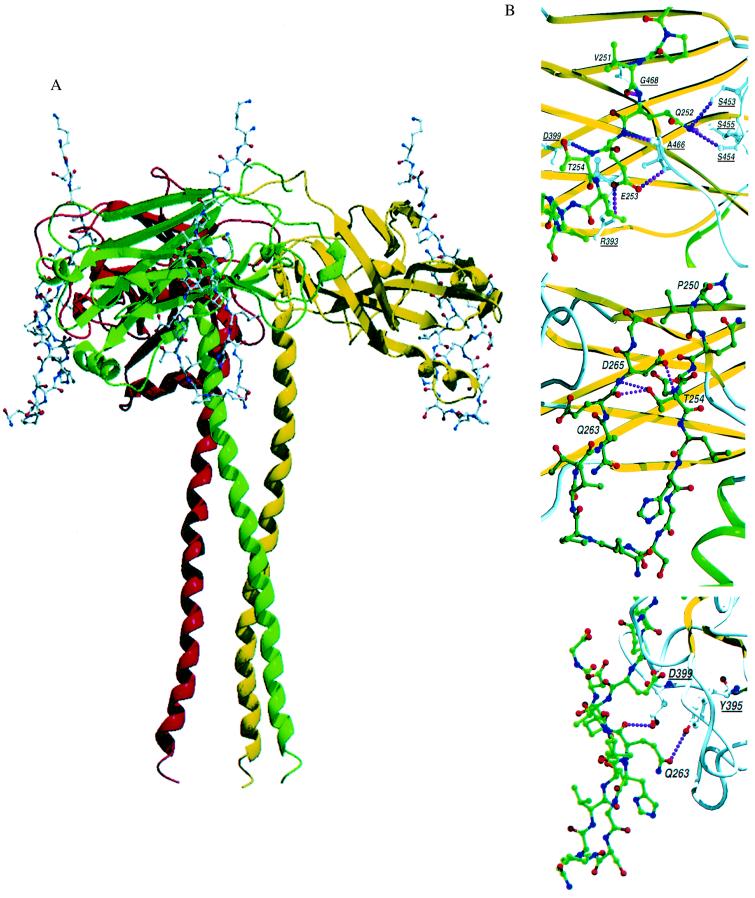
Figure 3.
TRAF3/CD40 interactions. (A) Schematic drawing of the TRAF3 trimer with the polypeptide backbone of TRAF3 presented as a ribbon model and the CD40 peptide shown as a ball and stick model. One CD40 fragment binds to each TRAF3 monomer at the edge of the TRAF3 domain, crossing one β-sheet. No conformational changes were seen when comparing TRAF3 alone or bound to CD40. (B) Close-up view of the CD40 fragment bound to TRAF2 and TRAF3. Residues in CD40 are labeled, and critical contact residues in TRAF3 are also marked and underlined for identification. Interactions within 3.0 Å that are proposed to dictate specific recognition of CD40 and TRAF3 or TRAF2 are shown as dotted lines. The images show intramolecular hydrogen bonds within the CD40 fragment that stabilize the reverse turn (Middle) and direct contacts between CD40 and TRAF3 (Bottom). These images can be contrasted with the contacts in TRAF2 (Top; 1CZZ-; based on figure 3 of Ye et al. in ref. 34).