Figure 1.
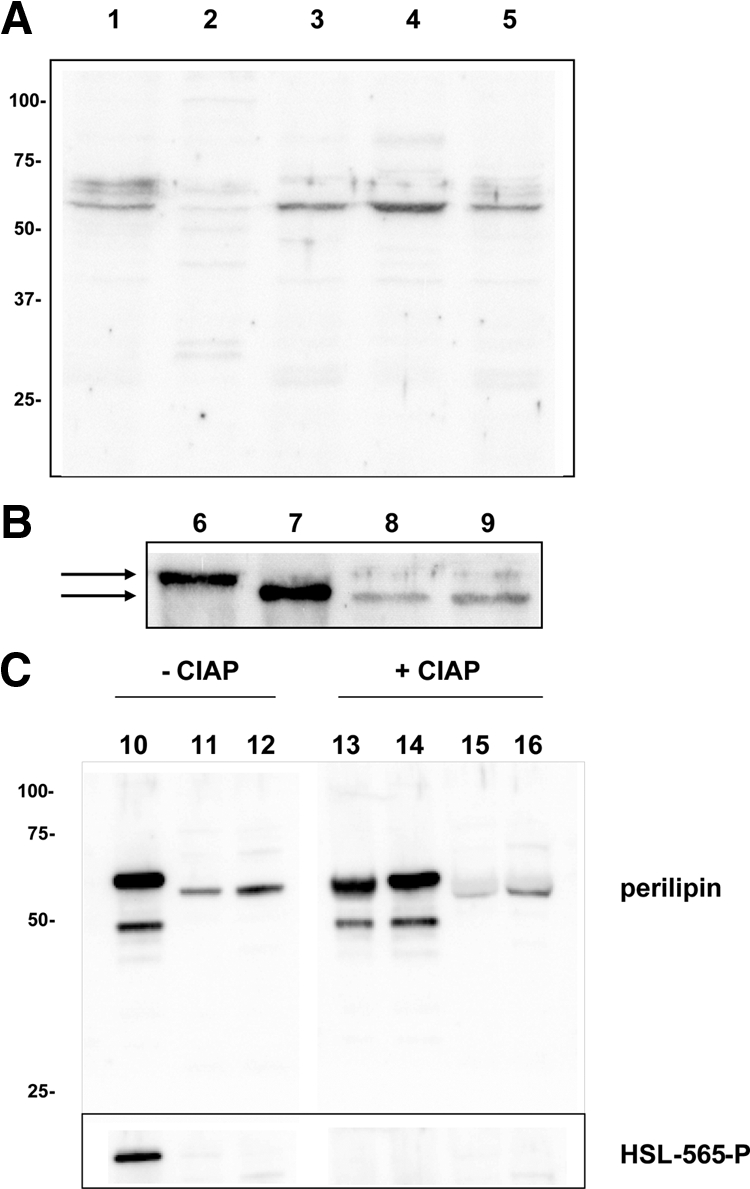
INS-1 cells express perilipin protein. A, Western blot analysis for perilipin after fractionation of INS-1 cells. Twenty micrograms of protein were loaded in each lane. Lane 1, INS-1 crude homogenate; lane 2, supernatant after 1 h of centrifugation at 100,000 × g; lane 3, the pellet to the supernatant in lane 2; lane 4, supernatant after detergent solubilization of the pellet from lane 3 and subsequent centrifugation; lane 5, the pellet to the supernatant in lane 4. B, Western blot analysis for perilipin in rat WAT, rat pancreatic islets, and INS-1 cells. Four micrograms of total protein were loaded in lanes 6 and 7 and 20 μg in lane 8 and 9. Lane 6, Visceral WAT; lane 7, INS-1 cells overexpressing perilipin A; lane 8, detergent-solubilized supernatant from homogenized and fractionated pancreatic islets; lane 9: detergent-solubilized supernatant from homogenized and fractionated INS-1 cells. The arrows indicate the difference in apparent molecular size observed between perilipin from WAT and islet cells. C, Western blot analysis for perilipin and for Ser-565 phosphorylated HSL in rat WAT, rat pancreatic islets, and INS-1 cells after treatment or not with calf intestinal alkaline phosphatase (CIAP). A homogenate of WAT corresponding to 2 μg of total protein was treated with either 10 U (lane 13; 5 U/μg protein; 0.1 U/μl) or 2 U CIAP (lane 14; 1 U/μg protein; 0.02 U/μl) and homogenates of islets (lane 15) and INS-1 cells (lane 16) corresponding to 10 μg of total protein were treated with 10 U CIAP (1 U/μg protein; 0.1 U/μl) and analyzed together with samples not treated with CIAP (lane 10, WAT; lane 14, rat islets; lane 15, INS-1 cells) by Western blot. To confirm the efficiency of the CIAP treatment, the blot was also probed with a phosphospecific HSL antibody, which recognized Ser-565 phosphorylation on HSL in WAT in samples not treated with CIAP but not in CIAP-treated samples. HSL expression in islets and INS-1 cells was not detected using this antibody.
