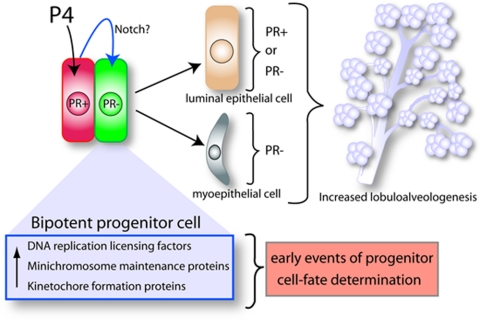
Figure 1.
An emerging model for progesterone action in the human breast shares common features with a proposed paracrine mechanism of action model for progesterone in the rodent mammary gland (24,25,26). Based on findings of Graham et al., (21), the schematic outlines a hypothetical model to explain the role of progesterone (P4) signaling in the expansion and morphogenesis of the epithelial compartment in the human breast. A subset of mammary epithelial cells (red), which are PR positive, receive, transduce, and subsequently relay, in a paracrine fashion, the proliferative signal imparted by P4 hormone to a juxtacrine cell subgroup (green) that is PR negative. Gene profiling studies implicate the notch pathway as possibly one of a number of paracrine signals that project the proliferative effects of progesterone from PR-positive cells to a subset of PR-negative cells (green) that exhibit bipotent progenitor properties. Upon receiving paracrine signal inputs, the progenitor cell subtype reprograms and proliferates to generate both luminal and myoepithelial cell lineages. Luminal epithelial descendants can be positive or negative for PR expression, whereas myoepithelial cell are PR negative. Together, the expansion and differentiation of these cell lineages contribute to increased lobuloalveologenesis. Induction of DNA replication licensing factors, minichromosome maintenance proteins, and kinetochore formation proteins are possible early molecular changes that precede progesterone-induced progenitor cell reprogramming and subsequent proliferation.