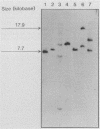
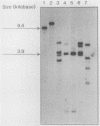
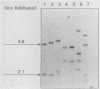
Abstract
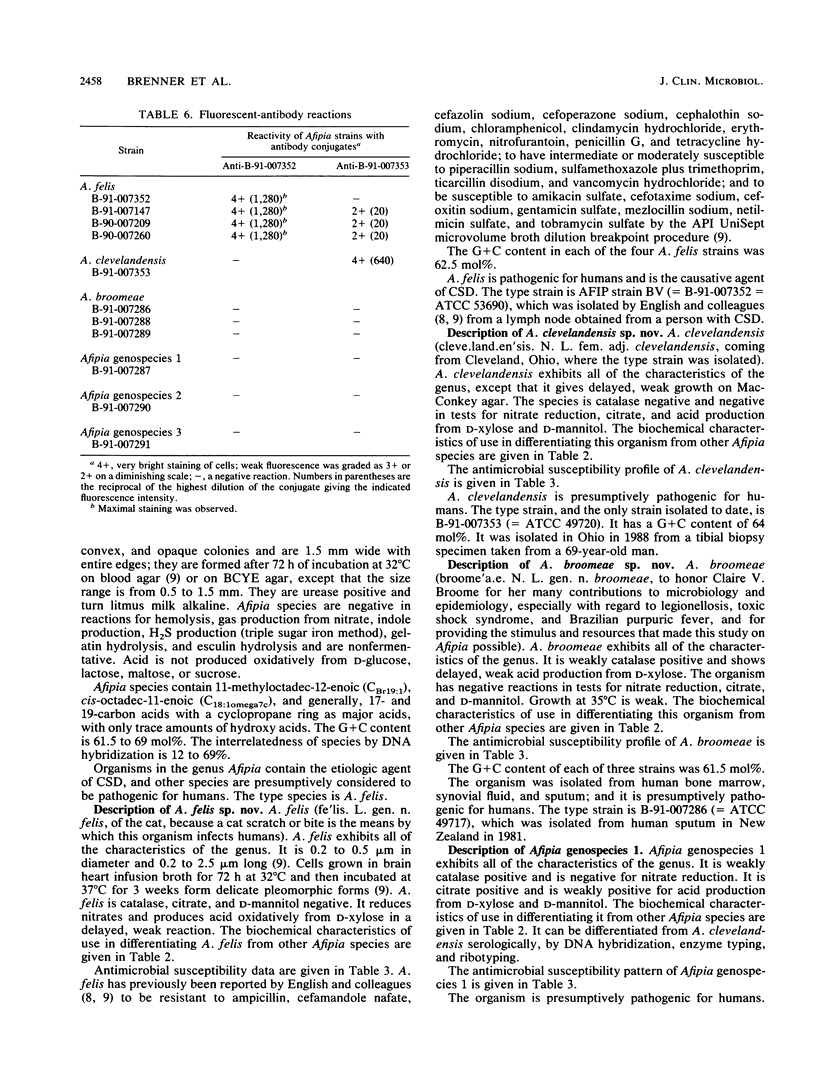
On the basis of phenotypic characterization and DNA relatedness determinations, the genus Afipia gen. nov., which contains six species, is described. The type species is Afipia felis sp. nov. (the cat scratch disease bacillus). Afipia clevelandensis sp. nov., Afipia broomeae sp. nov., and three unnamed not associated with cat-borne disease. All but one strain (Afipia genospecies 3) were isolated from human wound and respiratory sources. All Afipia species are gram-negative, oxidase-positive, nonfermentative rods in the alpha-2 subgroup of the class Proteobacteria. They are motile by means of a single flagellum. They grow on buffered charcoal-yeast extract agar and nutrient broth, but rarely on MacConkey agar, at 25 and 30 degrees C. They are urease positive; but they are negative in reactions for hemolysis, indole production, H2S production (triple sugar iron agar), gelatin hydrolysis, esculin hydrolysis, and peptonization of litmus milk. They do not produce acid oxidatively from D-glucose, lactose, maltose, or sucrose. The major cell wall fatty acids are 11-methyloctadec-12-enoic (CBr19:1), cis-octadec-11-enoic (C18:1omega7c), and generally, 9,10-methylenehexadecanote and 11,12-methyleneoctadecanoate; and there are only trace amounts of hydroxy acids. The guanineplus-cytosine content is 61.5 to 69 mol%. A. felis is positive for nitrate reduction and is delayed positive for acid production from D-xylose, but it is catalase negative. A. clevelandensis is negative in all of these tests. A. broomeae is weakly positive for catalase production and acid production from D-xylose, but it is negative for nitrate reduction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. J., McWhorter A. C., Knutson J. K., Steigerwalt A. G. Escherichia vulneris: a new species of Enterobacteriaceae associated with human wounds. J Clin Microbiol. 1982 Jun;15(6):1133–1140. doi: 10.1128/jcm.15.6.1133-1140.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry W. B., Pittman B., Harris P. P., Hebert G. A., Thomason B. M., Thacker L., Weaver R. E. Detection of Legionnaires disease bacteria by direct immunofluorescent staining. J Clin Microbiol. 1978 Sep;8(3):329–338. doi: 10.1128/jcm.8.3.329-338.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahoussaye P. M., Osborne B. M. Cat-scratch disease presenting as abdominal visceral granulomas. J Infect Dis. 1990 Jan;161(1):71–78. doi: 10.1093/infdis/161.1.71. [DOI] [PubMed] [Google Scholar]
- English C. K., Wear D. J., Margileth A. M., Lissner C. R., Walsh G. P. Cat-scratch disease. Isolation and culture of the bacterial agent. JAMA. 1988 Mar 4;259(9):1347–1352. doi: 10.1001/jama.259.9.1347. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Koehler J. E., LeBoit P. E., Egbert B. M., Berger T. G. Cutaneous vascular lesions and disseminated cat-scratch disease in patients with the acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. Ann Intern Med. 1988 Sep 15;109(6):449–455. doi: 10.7326/0003-4819-109-6-449. [DOI] [PubMed] [Google Scholar]
- LeBoit P. E., Berger T. G., Egbert B. M., Yen T. S., Stoler M. H., Bonfiglio T. A., Strauchen J. A., English C. K., Wear D. J. Epithelioid haemangioma-like vascular proliferation in AIDS: manifestation of cat scratch disease bacillus infection? Lancet. 1988 Apr 30;1(8592):960–963. doi: 10.1016/s0140-6736(88)91779-5. [DOI] [PubMed] [Google Scholar]
- Lenoir A. A., Storch G. A., DeSchryver-Kecskemeti K., Shackelford G. D., Rothbaum R. J., Wear D. J., Rosenblum J. L. Granulomatous hepatitis associated with cat scratch disease. Lancet. 1988 May 21;1(8595):1132–1136. doi: 10.1016/s0140-6736(88)91952-6. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Margileth A. M., Wear D. J., English C. K. Systemic cat scratch disease: report of 23 patients with prolonged or recurrent severe bacterial infection. J Infect Dis. 1987 Mar;155(3):390–402. doi: 10.1093/infdis/155.3.390. [DOI] [PubMed] [Google Scholar]
- McClelland M., Jones R., Patel Y., Nelson M. Restriction endonucleases for pulsed field mapping of bacterial genomes. Nucleic Acids Res. 1987 Aug 11;15(15):5985–6005. doi: 10.1093/nar/15.15.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney R. M., Thacker L., Harris P. P., Lewallen K. R., Hebert G. A., Edelstein P. H., Thomason B. M. Four serogroups of Legionnaires' disease bacteria defined by direct immunofluorescence. Ann Intern Med. 1979 Apr;90(4):621–624. doi: 10.7326/0003-4819-90-4-621. [DOI] [PubMed] [Google Scholar]
- McKinney R. M., Thomason B. M., Harris P. P., Thacker L., Lewallen K. R., Wilkinson H. W., Hebert G. A., Moss C. W. Recognition of a new serogroup of Legionnaires disease bacterium. J Clin Microbiol. 1979 Jan;9(1):103–107. doi: 10.1128/jcm.9.1.103-107.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Holzer G., Wallace P. L., Hollis D. G. Cellular fatty acid compositions of an unidentified organism and a bacterium associated with cat scratch disease. J Clin Microbiol. 1990 May;28(5):1071–1074. doi: 10.1128/jcm.28.5.1071-1074.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor S. P., Dorsch M., Steigerwalt A. G., Brenner D. J., Stackebrandt E. 16S rRNA sequences of Bartonella bacilliformis and cat scratch disease bacillus reveal phylogenetic relationships with the alpha-2 subgroup of the class Proteobacteria. J Clin Microbiol. 1991 Oct;29(10):2144–2150. doi: 10.1128/jcm.29.10.2144-2150.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves M. W., Evins G. M., Heiba A. A., Plikaytis B. D., Farmer J. J., 3rd Clonal nature of Salmonella typhi and its genetic relatedness to other salmonellae as shown by multilocus enzyme electrophoresis, and proposal of Salmonella bongori comb. nov. J Clin Microbiol. 1989 Feb;27(2):313–320. doi: 10.1128/jcm.27.2.313-320.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T. D., Chalgren S. Medium for use in antibiotic susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1976 Dec;10(6):926–928. doi: 10.1128/aac.10.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]