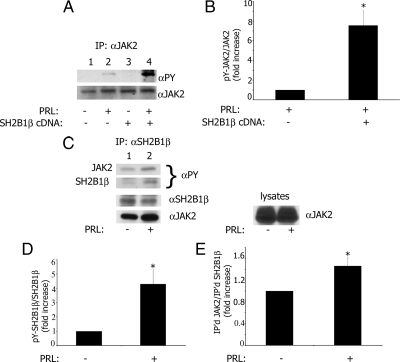
Figure 6.
SH2B1β enhances tyrosyl phosphorylation of JAK2 in response to prolactin. A, SH2B1β was either overexpressed (lanes 3 and 4) or was not (lanes 1 and 2) in T47D cells. The cells were deprived of serum and treated without (lanes 1 and 3) or with (lanes 2 and 4) 150 ng/ml PRL for 20 min. JAK2 was immunoprecipitated with α-JAK2, immunoblotted with α-PY, and reblotted with α-JAK2. B, The graph represents densitometric analysis of the bands obtained for tyrosyl-phosphorylated JAK2 normalized with total JAK2. Bar represents mean ± se. *, P < 0.05 compared with cells not expressing SH2B1β; n = 3. C, PRL promotes association of SH2B1β with JAK2 and stimulates tyrosyl phosphorylation of SH2B1β. T47D cells were treated with (lane 2) or without (lane 1) PRL as above, and endogenous SH2B1β was immunoprecipitated with α-SH2B1β, immunoblotted with α-PY, and reblotted with α-JAK2 and α-SH2B1β. D, The graph represents densitometric analysis of the bands obtained for tyrosyl-phosphorylated SH2B1β normalized with total SH2B1β. Bar represents mean ± se. *, P < 0.05 compared with cells not treated with PRL; n = 3. E, The graph represents densitometric analysis of the bands obtained for co-immunoprecipitated JAK2 normalized with amount of immunoprecipitated SH2B1β. Bar represents mean ± se. *, P < 0.05 compared with cells not treated with PRL; n = 3.