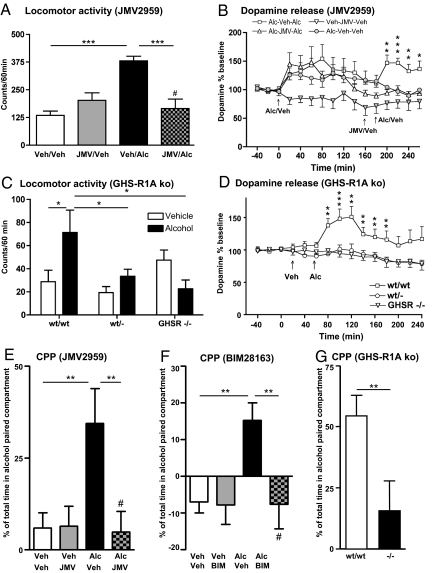
Fig. 2.
Suppressed ghrelin signaling, by either ghrelin receptor (GHS-R1A) antagonist (JMV2959) or GHS-R1A knockout mice, attenuates alcohol (Alc) reward. (A) Alcohol-induced locomotor stimulation was attenuated by a single i.p. injection of JMV2959 [but not by vehicle (Veh) injection] to NMRI mice [F (3, 28) = 12.86, P = 0.001] (n = 8 in each group; ***, P < 0.001, #, P = n.s. for Veh–Veh vs. JMV–Alc, Bonferroni post hoc test). (B) The alcohol-induced increase in accumbal dopamine release was absent in GHS-R1A antagonist (JMV2959, i.p.)-mice but not in vehicle-treated NMRI mice (n = 8 in each group). In B we first demonstrated a significant effect of alcohol to increase dopamine release in comparison to vehicle treatment [treatment F (1, 14) = 0.01, P = 0.65; time F (10, 140) = 4.34, P = 0.001; treatment × time interaction F (10, 140) = 0.79, P = 0.64]. Second, we showed that pretreatment with JMV2959 (i.p.) attenuated the alcohol-induced increase in dopamine release compared to vehicle pretreatment [treatment F (1, 14) = 14.12, P = 0.002; time F (5, 70) = 2.38, P = 0.05; treatment × time interaction F (5, 70) = 1.13, P = 0.35]. This difference (P < 0.001) was evident at time intervals 200, 220, 240, and 260 min (*, P < 0.05; **, P < 0.01; ***, P < 0.001, Bonferroni post hoc test). (C) The acute locomotor stimulation of alcohol observed in wild-type mice was abolished in both heterozygous and homozygous GHS-R1A knockout mice [F (5, 56) = 4.08, P = 0.003]. No difference was found in the locomotor response between any of the genotypes treated with vehicle, indicating that deletion of GHS-R1A does not affect activity per se (wt/wt: n = 8 for Veh and n = 6 for Alc; wt/−: n = 17 for Veh and n = 16 for Alc; −/−: n = 8 for Veh and n = 7 for Alc; *, P < 0.05; Tukey/Kramer). (D) The alcohol-induced increase in extracellular accumbal dopamine release was attenuated in both heterozygous (n = 13) and homozygous GHS-R1A knockout mice (n = 11) compared to wild-type mice (n = 8) [treatment F (2, 29) = 9.55, P = 0.001; time F (14, 406) = 4.65, P = 0.001; treatment × time interaction F (28, 406) = 2.79, P = 0.001]. This difference (P < 0.001) was observed at the time intervals 80, 100, 120, 140, 160, and 180 min (**, P < 0.01; ***, P < 0.001; Bonferroni post hoc test). (E) The alcohol-induced CPP was attenuated by an acute single i.p. injection of the GHS-R1A antagonist, JMV2959, in NMRI mice [F (3, 27) = 4.96, P = 0.01]. An alcohol-induced CPP in mice pretreated with vehicle (n = 7) was obtained and pretreatment with JMV2959 (n = 8) blocked this stimulation. No significant difference was observed between vehicle–vehicle (n = 8) and JMV2959-alcohol treatment (**, P < 0.01; #, P = n.s. for Veh–Veh vs. JMV–Alc, Bonferroni post hoc test). No effect of JMV2959 per se was observed (n = 8). (F) The alcohol-induced CPP was attenuated by an acute central administration of the GHS-R1A antagonist, BIM28163 (i.c.v.), in NMRI mice [F (3, 23) = 4.98, P = 0.01]. An alcohol-induced CPP in mice pretreated with vehicle (n = 7) was obtained and pretreatment with BIM28163 (n = 6) blocked this stimulation. No significant difference was observed between vehicle–vehicle (n = 6) and BIM28163-alcohol treatment (**, P < 0.01; #, P = n.s. for Veh–Veh vs. BIM–Alc, Bonferroni post hoc test). No effect of BIM28163 per se was observed (n = 8). (G) Alcohol induces a significant CPP in wild-type mice (n = 8), but not in GHS-R1A knockout mice (n = 5) [F (1, 11) = 5.15, P = 0.04]. A significant difference between CPP in wild type compared to GHS-R1A knockout mice was obtained (**, P < 0.01, Bonferroni post hoc test). All values represent mean ± SEM.