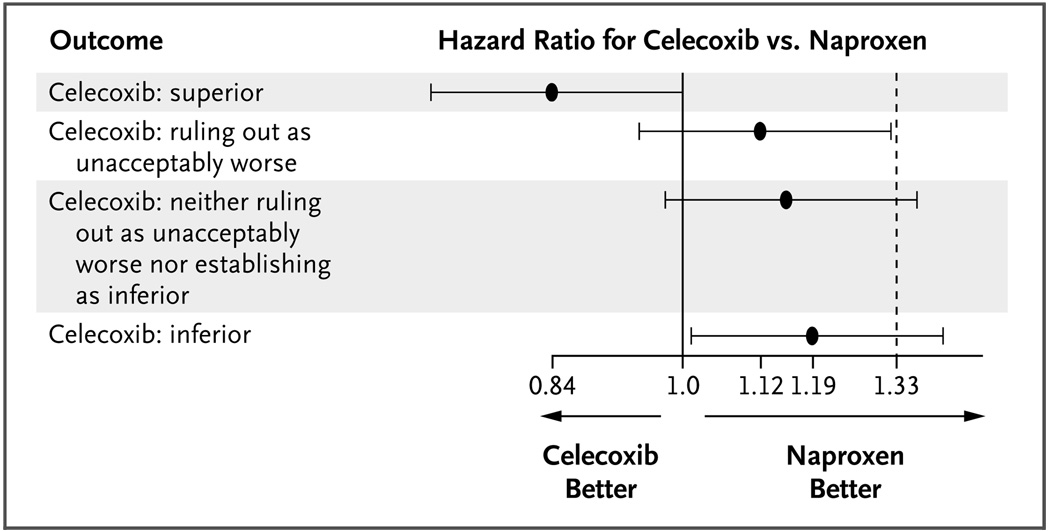
Figure 1. Hazard Ratios for the Composite Safety End Pont of Death from Cardiovascular Causes, Myocardial Infarction, or Stroke in the Celecoxib Group, as Compared with the Naproxen Group, According to Possible Outcomes in an Ongoing Trial.
The graph shows four possible outcomes in the PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety versus Ibuprofen or Naproxen) trial, which is being conducted to assess whether investigators could rule out an unacceptable increase in safety risks associated with celecoxib, as compared with naproxen. The black circles represent the point estimates, and the horizontal lines the 95% confidence intervals, for the risk of the composite safety end point with celecoxib, as compared with naproxen. In essence, if the entire confidence interval for the hazard ratio lies to the left of 1.33, the trial is “positive” in that investigators can rule out the possibility that the risk of the composite safety end point is 33% greater in the celecoxib group than in the naproxen group. If the entire confidence interval lies to the left of 1.0, celecoxib is superior to naproxen with regard to the risk of the composite safety end point, and if the entire confidence interval lies to the right of 1.0, celecoxib is inferior to naproxen. Data are from Fleming.7