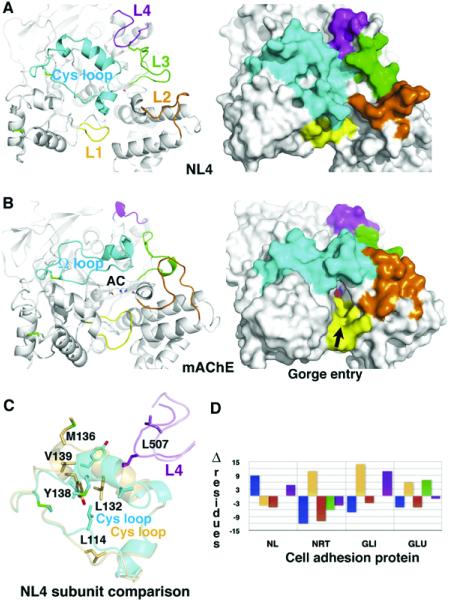
Figure 4. Comparison of the Surface Loops in NL4 and mAChE.
(A) The Cys-loop (Cys110-Cys146; cf. Figure 1) in NL4 and (B) the U-loop (Cys69-Cys96) in mAChE are displayed in cyan, loops L1 (Thr332-Ile338 in NL4) in yellow, loops L2 (Asp381-Val390) in orange, loops L3 (Gln477-Ser487) in green, and loops L4 (Gly503-Ser513) in magenta. The Cys-loop conformation is stabilized by several polar and nonpolar interactions between Leu120, His121, Asp122, and Trp127 at the loop edge, and Ser176, Glu179, Lys378, Gly490, and Leu507, which surround the central pocket. Label AC on mAChE denotes the active center. Molecular surfaces of NL4 ([A], right) and mAChE ([B], right) show the occluded surface in NL4 and the accessible gorge entry, marked by the arrow, in mAChE. (C) Superimposition of the two Cys-loops (cyan and orange) and interacting loops L4 (magenta and violet) from the two NL4 subunits in the dimer. Residues that undergo large displacements are displayed. (D) Graph of differences in the loop sizes (in numbers of residues) of NL4, neurotactin (NRT), gliotactin (GLI), and glutactin (GLU) compared with mAChE (color codes as in [A]).