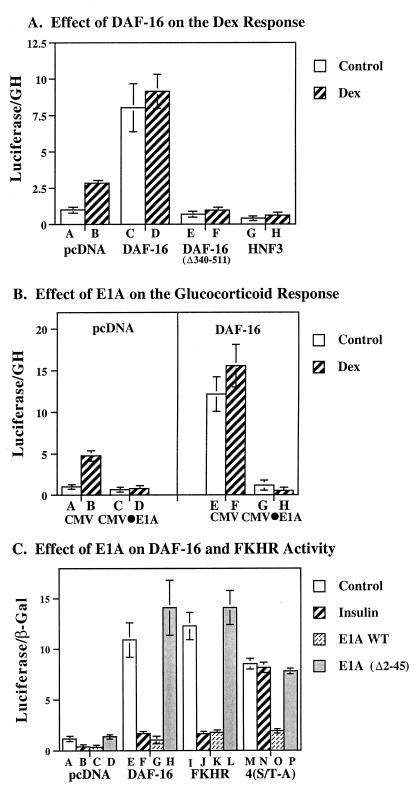
Figure 2.
(A) Effect of DAF-16 and HNF3α on insulin-responsive gene transcription. HepG2 cells were incubated in the presence or absence of insulin (1 milliunit/ml) for 16 h before harvesting. Luciferase activity recovered in the presence of pcDNA (bars A and B), pcDNA⋅DAF-16 (bars C and D), pcDNA⋅DAF-16 (Δ340–511) (bars E and F), and HNF3α (bars G and H) is shown. (B) Effect of E1A on dexamethasone-responsive gene transcription. Cells were transfected with the native IGFBP-1 promoter driving luciferase gene expression (15 μg/ml), and expression vectors including pcDNA alone (bars A–D) or pcDNA⋅DAF-16 (bars E–H); and CMV alone (1 μg/ml) (bars A, B, E, and F); or CMV⋅E1A (bars C, D, G, and H). Cells were inoculated with (bars B, D, F, and H) and without (bars A, C, E, and G) dexamethasone (0.5 μM) for 18 h. Luciferase activity is shown corrected for growth hormone (GH) and normalized to the control value for pcDNA alone. (C) DAF-16 gene expression is inhibited by insulin and by wild-type E1A but not by E1A Δ2–36. HepG2 cells were transiently cotransfected with the native IGFBP-1 promoter-luciferase gene (10 μg/ml), and the pcDNA expression vector alone (1 μg/ml) (bars A–D), or wild-type pcDNA⋅DAF-16 (bars E–H), or pcDNA⋅FKHR (bars I–L) or pcDNA⋅DAF-16 4(S/T-A) (bars M–P). Control and insulin-stimulated activity was assessed in the presence of the expression vector CMV alone (0.2 μg/ml; bars A, B, E, F, I, J, M, and N). The effect of wild-type CMV⋅E1A (bars C, G, K, and O) or a derivative of E1A that fails to interact with CBP, CMV⋅E1A Δ2–36 (bars D, H, L, and P) is shown.