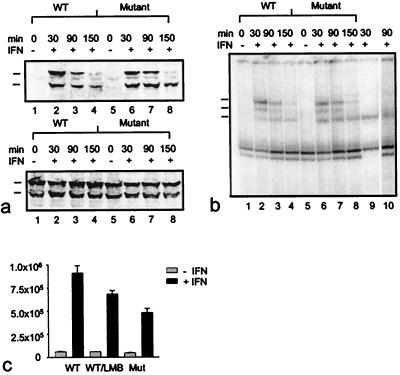
Figure 6.
Kinetics of tyrosine phosphorylation (a), DNA binding activity (b), and transcriptional activation of an IFNγ-responsive luciferase reporter gene (c) displayed by wild-type and export mutant Stat1. (a) 293T cells were transiently transfected with wild-type or Leu308,312Ala double mutant GFP-Stat1 DNA and pooled. Subsequently, equal numbers of cells were plated and 15 h later treated with or without IFNγ for 30 min. At this time point (30 min) as well as 1 hour (90 min) and 2 hours (150 min) later, the cells were lysed, and nuclear extracts were collected. Nuclear proteins were resolved on SDS/PAGE gels and blotted. Shown is a Western blot with a phosphospecific Stat1-Tyr701 antibody (Upper) and a reprobing with Stat1 antibody (Lower). The positions of the transfected proteins (upper mark) and the endogenous Stat1 (lower mark) are indicated. (b) Nuclear extracts prepared from the same cells described in a were subjected to DNA-binding analysis. Extracts were incubated with radiolabeled M67 probe and separated on 4% nondenaturing polyacrylamide gels. The positions of homodimers of endogenous Stat1 (bottom mark), of homodimers of transfected GFP-constructs (top mark), and of heterodimers of native and recombinant Stat1 proteins (middle mark) are indicated at the left edge of the gel. In lane 9, the DNA-binding pattern of interferon-stimulated (30 min) untransfected 293T cells is shown. Supershifting with a polyclonal GFP antibody (CLONTECH) of the same extract used in lane 7 specifies GFP-containing complexes (lane 10). (c) Effects of Leu308,312Ala mutations and LMB on transcription activation. Wild-type (WT) and mutant proteins (Mutant) were coexpressed with an IFNγ-inducible reporter plasmid in U3A cells. Luciferase activity was determined 36 h posttransfection in cells that had been stimulated with or without IFNγ for 6 h in the presence or absence of LMB. Error bars represent standard deviations for four independent experiments.