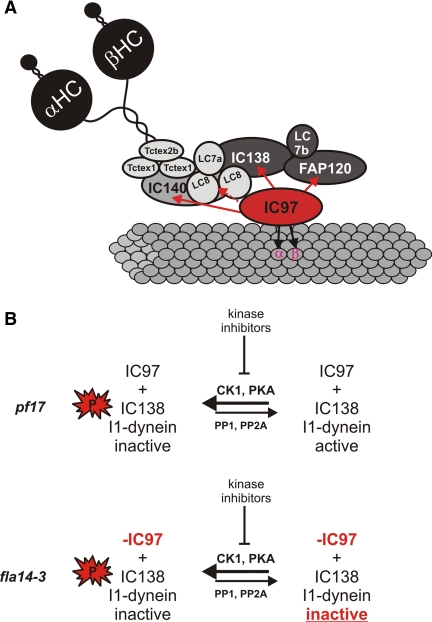
Figure 6.
Models for I1-dynein structural interactions and for control of I1-dynein activity. (A) The I1-dynein interaction map showing the IC97 interactions determined in this study. IC97 interacts with IC140 based on analysis of the ida7-1::IC140 5A mutant; IC97 interacts directly with IC138 based on analysis of the bop5-2 mutant; biochemical data indicate a direct interaction between IC97 and α- and β-tubulin; and IC97 may interact with the LC8 dimer (dashed line) based on our analysis of the fla14-3 mutant, although we hypothesize that IC97 does not directly interact with LC8. In addition, FAP120 may interact with IC97 and the C terminus of IC138 (based on the bop5-1 and fla14-3 mutants). Interactions involving the LC subunits have not been fully determined, other than the LC7b-IC138 interaction (Dibella et al., 2004a; Hendrickson et al., 2004). (B) A model for regulation of I1-dynein activity showing the requirement of IC97 for I1-dynein activity is shown. In the presence of IC97, such as in pf17 axonemes, I1-dynein can be dephosphorylated (extrinsically with kinase inhibitors) and subsequently activated. However in the absence of IC97 (such as in fla14-3), I1 dynein remains inactive even though IC138 dephosphorylation occurs.