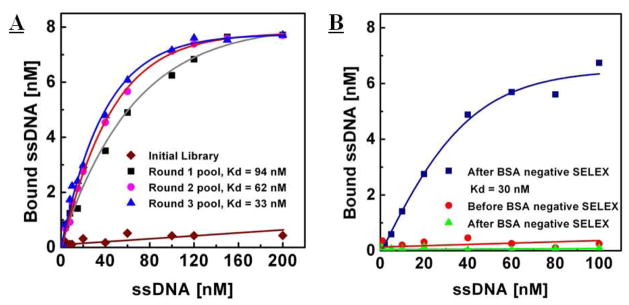
Figure 4.
Fluorescence measurements to determine dissociation constants (Kd) of selected aptamers. A) The initial, naïve library exhibits negligible binding to the streptavidin target  ). After the first round of selection, the average Kd of the enriched pool was 94 ± 10 nM (■). Subsequently, second and third round selections yielded an average Kd of 62 ± 5 nM (
). After the first round of selection, the average Kd of the enriched pool was 94 ± 10 nM (■). Subsequently, second and third round selections yielded an average Kd of 62 ± 5 nM ( ) and 33 ± 5 nM (
) and 33 ± 5 nM ( ), respectively. B) After a single round of negative selection against BSA, the aptamer pool exhibited slightly higher affinity for streptavidin (Kd = of 30 ± 5 nM,
), respectively. B) After a single round of negative selection against BSA, the aptamer pool exhibited slightly higher affinity for streptavidin (Kd = of 30 ± 5 nM,  ). In contrast, the affinity for BSA significantly decreased 5-fold from (
). In contrast, the affinity for BSA significantly decreased 5-fold from ( ) to (
) to ( ).
).