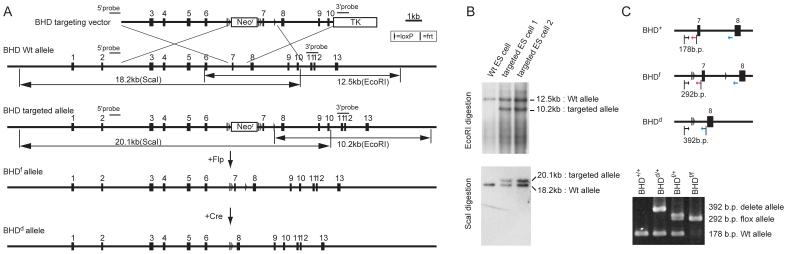
Fig. 1. Generation of conditional BHD knockout mice.
A) Targeting strategy. Birt-Hogg-Dube’gene (BHD) targeting vector was constructed by recombineering methodology using homologous recombination (27). A neomycin resistance (Neor) cassette flanked by Frt (bar) and loxP (triangle) sequences was inserted into intron 6 for positive selection, and the thymidine kinase gene was included for negative selection. A second loxP sequence was inserted into intron 7. Correctly-targeted embryonic stem (ES) cells were identified by Southern blot analysis and injected into blastocysts to produce chimeras. Backcrossing to C57BL/6 mice produced heterozygous F1 offspring with germline transmission of the BHD floxed (f)-Neo allele. The Neo cassette flanked by Frt sites was excised in vivo by crossing with mice expressing the Flp recombinase transgene under the ubiquitous β-actin promoter. To produce the BHD deleted (d) allele, BHD f/+ mice were crossed with mice expressing the Cre recombinase transgene under the ubiquitous β-actin promoter (24). Deletion of exon 7 resulted in a frame shift and premature termination codon in exon 8, which caused mRNA degradation by the nonsense-mediated decay mRNA surveillance system (43). B) The targeted ES cells were screened by Southern blotting of EcoRI and ScaI digested DNA using two different external probes located outside the targeting sequence as shown in (A). C) Polymerase chain reaction (PCR)-based genotyping was performed using DNA extracted from mouse tails for routine monitoring of inheritance in offspring. Locations of PCR primers are indicated by arrows.