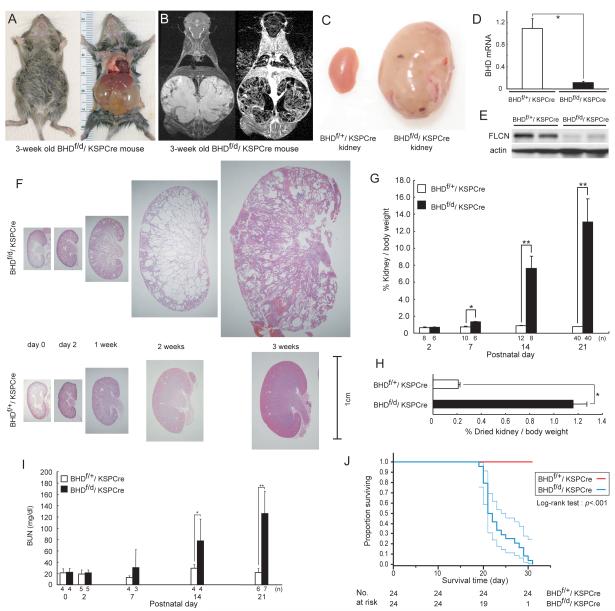
Fig. 2. Phenotypic features of BHD-targeted deletion in the kidney.
A) Gross picture of a 3-week old BHDf/d/KSP (cadherin 16 kidney-specific promoter)-Cre mouse shows a distended abdomen (left panel). The large cystic kidneys fill the abdominal cavity (right panel) and are found in 100% of BHDf/d/KSP-Cre mice. B) T2 weighted coronal magnetic resonance imaging (MRI) image (left) and corresponding Gadolinium-enhanced T1 weighted dynamic subtraction MRI image (right) of a 3-week-old BHDf/d/KSP-Cre mouse show enlarged cystic kidneys with reticular interstitium and delayed excretion of contrast medium. C) Comparison of gross features of 3-week-old control BHDf/+/KSPCre and knockout BHDf/d/ KSP-Cre kidneys reveals a normal phenotype in control mice. Knockout BHDf/d/ KSP-Cre kidneys show symmetric enlargement without focal masses. One representative image of 40 mice for each genotype is shown. D) BHD mRNA expression in kidneys of 3-week-old mice was quantified by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) using exon 6 and 7 amplification. Three mice of each genotype were analyzed. Data are presented as means and 95% confidence intervals from three independent experiments performed in triplicate (Welch’s t test:*P<.001). E) Folliculin (FLCN) expression in control and knockout kidneys was estimated by immunoblotting. One representative of three independent experiments performed in two mice is shown. F) Comparison of BHD control and knockout kidney histology (hematoxylin and eosin staining) at different ages shows no obvious differences at days 0 and 2. At 1 week, BHD-knockout mice have enlarged kidneys with dilated collecting ducts and a few dilated cortical tubules. By 2 weeks of age, most collecting ducts in the medulla are markedly dilated. The entire BHD-knockout kidney is diffusely filled with cystic collecting ducts and tubules at 3 weeks of age, and at this point anatomic distinction between cortex and medulla is lost. One representative of at least three mice at each age is shown. G) Relative ratio of kidney to body weight (BW;100 X kidney weight/[BW—kidney weight]) was calculated at different ages. The mean kidney weight of the BHDf/d/KSP-Cre mice at 3 weeks of age was 13.1% of body weight compared with 0.77% for control mice (n=40 for each group, difference=12.3%, 95% CI=11.1% to 13.6%; Welch’s t test:P<.001). Data are represented as means and 95% confidence intervals (*P =.002, ** P <.001). H) Relative ratio of dried kidney to body weight (100 X dried kidney weight/[BW—wet kidney weight]) of 3-week-old mice was calculated. Even with loss of cystic fluid, the dry weight of BHDf/d/KSP-Cre kidneys was statistically significantly greater than control kidneys (mean=1.16%, n=8 versus mean=0.21%, n=10, difference=0.95%, 95% CI=0.84% to 1.06%; Welch’s t test: * P<.001). Data are represented as means and 95% confidence intervals. I) BHDf/d/KSP-Cre mice die of renal failure. Blood urea nitrogen (BUN) levels were determined at different ages. Statistically significant elevation of BUN levels was observed at 2 weeks of age in BHDf/d/KSP-Cre mice compared with littermate controls (n=4 for each group, mean=77.7 mg/dL versus mean=29.2mg/dL, difference=48.5mg/dL, 95% CI=18.6 to 78.3 , Welch’s t test:*P=.03). Similar statistically significant differences were seen between 3-week old BHDf/d/KSP-Cre mice (n=7) and control littermates (n=6) (mean=126.1 mg/dL versus mean=21.8 mg/dL, difference=104.3 mg/dL, 95% CI=64.9 to 143.9 , Welch’s t test:**P=.0012). Data are represented as means and 95% confidence intervals. J) Kaplan-Meier survival analysis shows a statistically significant difference between control and BHD knockout mice (log-rank test, P<.001). Median survival time of BHD knockout mice is 21.5 days (n=24).