Abstract
Objective:
To evaluate the anatomical and functional outcome in eyes with indocyanine green (ICG)-assisted idiopathic epiretinal membrane (ERM) peeling by optical coherence tomography (OCT) and multifocal electroretinogram (MFERG).
Design:
Prospective, interventional, noncomparative case series.
Methods:
Twenty eyes of 20 patients with idiopathic ERM underwent pars plana vitrectomy and ICG-assisted ERM and internal limiting membrane (ILM) removal. Visual acuity (VA), OCT, and MFERG measurements were performed preoperatively and postoperatively at 1, 3, 6, and 12 months.
Results:
Best-corrected VA (BCVA) improved ≥2 Snellen lines in 70% of our patients at the 12th postoperative month. Mean VA increased from 20/100 preoperatively to 20/40 at 12 months. VA increased significantly at all postoperative examinations, compared to preoperative VA. Foveal thickness measured by OCT decreased significantly at all postoperative examinations. OCT mean values dropped from 472.3 μm preoperatively, to 249.2 μm at 12 months. Preoperative MFERG values significantly improved only at 12 months. OCT measurements and MFERG values did not correlate at any time. OCT values correlated with VA values only preoperatively while MFERG measurements correlated with VA at 12 months.
Conclusions:
In our series of eyes with ERM surgery, OCT measurements and VA improved gradually throughout the first postoperative year, while MFERG values showed significant improvement at 12 months.
Introduction
Although the first histopathologic description of proliferative membranes on the surface of the retina was reported by Iwanoff in 1865 (Iwanoff 1865), it was not until 1978 that Machemer (Machemer 1978) described successful surgical delamination of epiretinal membranes. Since then a variety of intraocular tools are used to begin the delamination process, after pars plana vitrectomy. More recently, the use of intravitreal dye has been introduced for better visualization of the membrane. The use of indocyanine green (ICG) seems to be the most popular technique, despite the reports of possible toxicity or damage to the Muller cells (Haritoglou et al 2002).
Optical coherence tomography (OCT) is an imaging technique that obtains noninvasive cross-sectional images in vivo with a 10 μm resolution. OCT provides evidence of the anatomical status of the posterior pole of the eye. In patients with epiretinal membrane (ERM), OCT is useful not only in detecting the membrane, but additionally in studying the morphology of the macula, in distinguishing pseudoholes from full-thickness macular holes, and mainly in measuring preoperative and postoperative retinal thickness. Visual function before and after macular surgery is assessed by visual acuity measurement, which however is only a part of the impaired vision from ERM development. Seventy-five to ninety-three percent of patients with symptomatic ERM typically experience varying degrees of blurred vision and metamorphopsia (Hirokawa 1986). An objective assessment of the visual function can be made by multifocal electroretinogram (MFERG). MFERG selects the elecrophysiological responses of multiple retinal locations of the macular and perimacular area, which are tested simultaneously, allowing functional mapping of the central retina.
The purpose of this study was to compare anatomic and functional outcomes of eyes with ERM operated on by ICG-assisted vitrectomy using OCT and MFERG.
Methods
Study design: Prospective, interventional, noncomparative case series
Patients
Twenty eyes of 20 patients with idiopathic ERMs were examined prospectively before and after vitrectomy for one year. All surgery was performed at the Athens University Clinic of Ophthalmology between January 2004 and May 2005 by two experienced vitreoretinal surgeons.
The age of the patients ranged from 59 to 78 years. Nine patients were male and 11 female. All patients had grade 1 (crinkled cellophane maculopathy) and 2 (macular pucker) idiopathic ERMs according to Gass classification. No grade 0 (cellophane maculopathy) patients were included in the study.
Inclusion criteria
Patients with idiopathic ERM with significant loss of visual acuity (≤20/40). All patients were phakic, without cataract formation.
Exclusion criteria
Eyes with other macular pathology, history of ocular inflammation and previous ocular surgery and patients with systematic disorders affecting the eye, such as diabetes, were excluded from the study. Patients with ERM and lamellar hole were also excluded from the study.
The study protocol was submitted and approved by the Medical School of the University of Athens. All patients were informed for the procedures of the study and gave their written consent.
Methods
Patients were examined preoperatively and at 1, 3, 6, and 12 months postoperatively. Examination included slit-lamp examination, fundus examination, visual acuity measurement, OCT, and MFERG. Anatomic evaluation was determined with OCT and functional evaluation included visual acuity and MFERG (areas 1 and 2) examination. The preoperative data included lens status. The lens was examined preoperatively and postoperatively in order to determine cataract formation according to the Lens Opacities Classification System III (LOCS III) system.
For the OCT examination the Humphrey 2004 Stratus OCT Model 3000 (Humphrey Instruments, Carl Zeiss Meditec Inc., Dublin, California, USA) was used. Two experienced masked observers performed in all eyes macular thickness scanning, which consists of 6 radial tomograms of 6 mm length, centered through the fovea. Macular thickness at the fovea, from the retinal pigment epithelium to the inner retinal surface, was measured based on the standard OCT software for retinal map analysis which automatically calculates retinal thickness on the central area of the fovea and 8 surrounding areas. This program was considered more accurate for ERM measurements, since the patient’s fixation point in these cases may not be at the center of the fovea. Longitudinal resolution was 6–10 μm.
For the MFERG, the VERIS (Visual Evoked Response Imaging System) III Clinic System (Tomey Corp., Nagoya, Japan) was used. The stimulus matrix consisted of 61 hexagonal elements displayed on a CRT color monitor driven at a frame of 75 Hz. These hexagons elicit approximately equal signal amplitude at all locations at a normal retina. Each hexagon was independently alternated between black and white at a rate of 75 Hz, and this stimulation technique allowed a retinal response from each stimulus. The luminance of the stimulus for white was 200 cd/m2 and the contrast was 99.3%. The radius of the stimulus array subtended approximately 20 degrees high and 25 degrees wide. The bandwidth of the amplifier was 10 to 300 Hz (−6bdB/oct) and the amplification was ×10,000. Area 1 corresponds to the 2.8 central degrees of the retina and area 2 extends from 2.8 to 9 degrees from the center of the fovea. Area 1 corresponds roughly to the fovea. The value for area 2 is the mean value of the 6 hexagons which represent the parafoveal area. The normal ranges for these amplitudes were defined by calculation of the median and the 95% confidence intervals in one eye each of 30 volunteers aged 35–50 years. The average relative response density of retinal area corresponding to area 1 is roughly 20.0 nV/deg2 and to area 2 is 15.4 nV/deg2.
In all eyes the surgical technique consisted of pars plana vitrectomy and removal of the cortical vitreous from the posterior pole. Then ICG dye 0.5%, or of an osmolarity of 270 mOsm, was injected under liquid for 30 seconds in the vitreous cavity, and dyeing of the internal limiting membrane (ILM) peripherally to the ERM, which does not stain, was achieved. The ERM and the ILM were removed from the macular area, guided by the green dye. No tamponade was used in any case.
Anatomic success was defined by the OCT findings postoperatively when no evidence of ERM was found in the foveal area. Functional success was defined as the improvement of ≥2 Snellen lines of the best corrected visual acuity.
Statistical analysis
In order to compare the preoperative values of the best-corrected visual acuity (BCVA) with the postoperative BCVA at 1, 3, 6, and 12 months we performed nonparametric analysis because initial analysis showed that VA did not follow normal distribution. In order to compare preoperative with postoperative retinal thickness measurements by OCT as well as the values of MFERG area 1 and 2, for the same examination periods, we performed paired t-tests. Because of the small number of patients, nonparametric methods such as Wilcoxon signed-rank and Sign test were also used to fortify our results.
To examine if there was any correlation between the BCVA measurements and the OCT values, as well as the best-corrected visual acuities and the MFERG area 1 and 2 values, the Spearman analysis and regression analysis were used. The Spearman and regression analysis were also used to test whether there was any correlation between OCT measurements and MFERG values for area 1 and 2.
We studied as possible prognostic factors preoperative retinal thickness and preoperative VA in comparison to final visual acuity. For this purpose the patients were classified preoperatively in 2 groups for retinal thickness (>420 μm and ≤420 μm) and 2 groups for VA (>20/100 and ≤20/100). Logistics regression was performed to decide if mean VA preoperatively and at the end of the follow up period had statistically significant difference for the 2 groups.
Results
Anatomic success was achieved in all 20 patients confirmed by OCT (anatomical success rate 100%).
Table 1 summarizes the MFERG values (in nV/deg2), the OCT findings (in μm) and the VA measurements of our patients preoperatively and at 12 months.
Table 1.
MFERG values (in nV/deg2), OCT findings (in μm), and VA measurements of our patients preoperatively and at 12 months
|
MFERG preop |
MFERG postop 12m |
OCT preop | OCT postop 12 m | VA preop | VA postop 12 m | |||
|---|---|---|---|---|---|---|---|---|
| Area 1 | Area 2 | Area 1 | Area 2 | |||||
| 1 | 2.83 | 0.15 | 8.45 | 7.88 | 490 | 282 | 20/400 | 20/50 |
| 2 | 1.09 | 1.47 | 8.17 | 6.76 | 505 | 247 | 20/63 | 20/50 |
| 3 | 9.36 | 7.38 | 12.36 | 9.38 | 417 | 243 | 20/100 | 20/40 |
| 4 | 1.46 | 4.20 | 10.37 | 7.29 | 416 | 219 | 20/100 | 20/25 |
| 5 | 2.40 | 4.94 | 3.68 | 4.44 | 403 | 178 | 20/63 | 20/50 |
| 6 | 1.59 | 7.30 | 12.62 | 8.02 | 598 | 347 | 20/200 | 20/63 |
| 7 | 4.48 | 1.68 | 10.23 | 5.64 | 705 | 192 | 20/200 | 20/40 |
| 8 | 1.51 | 6.10 | 12.33 | 9.71 | 458 | 278 | 20/80 | 20/63 |
| 9 | 2.35 | 1.77 | 5.34 | 4.78 | 326 | 224 | 20/80 | 20/40 |
| 10 | 4.73 | 1.12 | 8.43 | 3.24 | 430 | 256 | 20/100 | 20/50 |
| 11 | 1.25 | 2.15 | 13.08 | 9.50 | 557 | 261 | 20/63 | 20/25 |
| 12 | 9.76 | 6.53 | 10.37 | 4.31 | 397 | 271 | 20/50 | 20/50 |
| 13 | 12.92 | 1.90 | 14.31 | 8.44 | 524 | 314 | 20/100 | 20/50 |
| 14 | 3.75 | 3.50 | 9.65 | 10.22 | 405 | 355 | 20/100 | 20/125 |
| 15 | 6.97 | 1.95 | 20.36 | 15.20 | 518 | 220 | 20/100 | 20/20 |
| 16 | 7.55 | 6.61 | 11.01 | 6.20 | 504 | 270 | 20/100 | 20/25 |
| 17 | 3.13 | 1.96 | 7.83 | 5.45 | 383 | 190 | 20/50 | 20/50 |
| 18 | 3.91 | 7.65 | 4.23 | 9.42 | 494 | 224 | 20/200 | 20/50 |
| 19 | 3.12 | 4.40 | 10.18 | 5.69 | 520 | 256 | 20/200 | 20/40 |
| 20 | 3.22 | 0.50 | 8.65 | 4.83 | 396 | 156 | 20/100 | 20/32 |
| MV | 4.37 | 3.61 | 10.08 | 7.32 | 472.30 | 249.15 | 20/100 | 20/40 |
| SD | 3.28 | 2.57 | 3.73 | 2.80 | 87.49 | 52.12 | ||
Abbreviations: MFERG, multifocal electroretinography; preop, preoperatively; postop, postoperatively; OCT, optical coherence tomography; VA, visual acuity; m, months; MV, Mean values; SD, standard deviation.
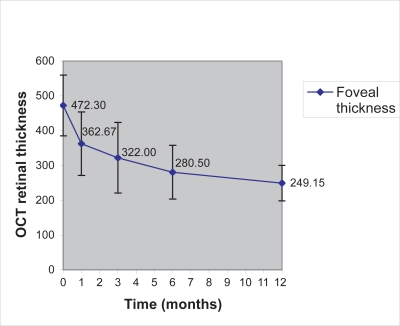
The thickness of the retina at the center of the fovea was found to range from 326 μm to 705 μm with a mean value of 472.30 μm (±87.49) preoperatively. Table 2 demonstrates the mean values and standard deviation of the OCT and MFERG measurements preoperatively and at 1, 3, 6, and 12 months postoperative examinations. In the 1st postoperative month, the retinal thickness was reduced and ranged from 221 to 531 μm (mean value 362.67 ± 91.24) which was statistically significant (p < 0.05), and decreased throughout the 12 postoperative months, as illustrated in Figure 1. In the one-year postoperative examination, the central retinal thickness measured by OCT ranged from 156 to 355 μm, with a mean value of 249.15 (±51.12) μm. Paired t-test and nonparametric methods demonstrated statistically significant difference (reduction) of retinal thickness preoperatively in comparison with postoperative examinations at 1, 3, 6, and 12 months. The percentage of patients with reduction in foveal thickness was 85% (17/20) in the 1st month, and 100% (20/20) in the 3rd, 6th, and 12th postoperative month examinations in comparison with preoperative values.
Table 2.
Mean values and standard deviation of the OCT and MFERG measurements preoperatively and at 1, 3, 6, and 12 months postoperative examinations
|
MFERG (MV-SD) |
OCT MV (SD) | VA (MV) | ||
|---|---|---|---|---|
| Area 1 | Area 2 | |||
| preop | 4.37 (3.27) | 3.61 (2.57) | 472.3 (87.49) | 20/100 |
| 1 m | 5.60 (2.51) | 3.76 (3.49) | 362.67 (91.24) | 20/71 |
| 3 m | 7.33 (3.54) | 4.90 (3.42) | 322.0 (101.44) | 20/54 |
| 6 m | 5.91 (2.64) | 5.50 (2.75) | 280.5 (77.36) | 20/45 |
| 12 m | 10.08 (3.73) | 7.32 (2.80) | 249.15 (51.12) | 20/40 |
Abbreviations: MV, mean value; SD, standard deviation; MFERG, multifocal electroretinography; OCT, optical coherence tomography; VA, visual acuity; preop, preoperatively; postop, postoperatively; m, months.
Figure 1.
Reduction of the OCT retinal thickness of our patients, measured in μm, throughout the follow-up period.
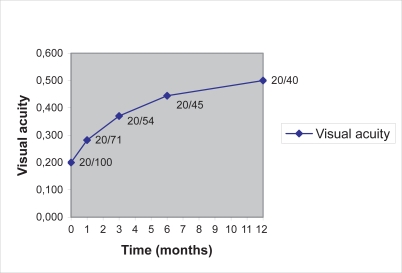
Preoperative BCVA ranged from 20/400 to 20/50 with a mean value of 20/100. Mean visual acuity improved in all postoperative examinations, as it is illustrated in Figure 2, and nonparametric methods (Wilcoxon signed-rank and Sign test) showed that there was statistically significant difference (increase) between the preoperative and the postoperative VA measurements at 1, 3, 6, and 12 months. In 14 of the 20 patients (70%) VA improved ≥2 Snellen lines in the 1st year. BCVA ranged from 20/125 to 20/20 at 12 months with a mean value of 20/40, as demonstrated in Table 1.
Figure 2.
Increase of the mean visual acuity of our patients throughout the follow-up period.
The thickness of the fovea measured preoperatively by OCT was found to be statistically related to the preoperative VA. Spearman correlation coefficient and also regression analysis showed a statistically significant negative correlation between these two variables (Spearman p = 0.028, regression analysis p = 0.02). There was no correlation of the foveal thickness measured by OCT postoperatively at 6 and 12 months and the VA respectively.
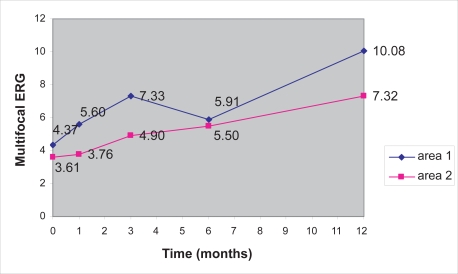
Preoperative visual function estimated by MFERG was poor, with decreased electrical activity at the foveal and parafoveal area. As it is demonstrated in Table 2, the mean retinal response density preoperatively was 4.37 nV/deg2 in area 1 and 3.61 nV/deg2 in area 2. Statistical analysis by paired t-test demonstrated that there was a significant increase between preoperative and 12 month postoperative MFERG values in area 1 (p = 0.0016 < 0.05), as illustrated in Figure 3. Because of the relatively small number of patients, nonparametric methods were also used, that came to the same conclusion (Wilcoxon signed-rank p = 0.0033 and Sign test p = 0.005). Statistically significant increase in MFERG area 2 values was also noticed at 12 months compared with preoperative values for area 2, as it is illustrated in Figure 3. This increase was confirmed by nonparametric methods (paired t-test p = 0.0081 < 0.05, Wilcoxon p = 0.0076 and Sign test p = 0.0327). Paired t-test showed no statistical difference between preoperative and postoperative measurements for area 2 for months 1, 3, and 6, which was confirmed by the nonparametric methods. Analysis also showed a borderline statistical difference of the preoperative and postoperative measurements at 3 months for area 1 (p = 0.045), but it was not confirmed by the nonparametric methods (Wilcoxon signed-rank p = 0.1386 and Sign test p = 0.2539).
Figure 3.
Increase of the MFERG (area 1 and area 2) values in the follow-up period.
Statistical analysis demonstrated that there was no statistically significant correlation between the values of MFERG (both for area 1 and 2) and the VA measurements between preoperative and postoperative examinations at 1, 3, and 6 months. Improvement of the MFERG values was noticed at the 12th postoperative month, when the mean retinal response density was 10.08 nV/deg2 in area 1 and 7.32 nV/deg2 in area 2 (Table 2). For VA and MFERG area1 correlation at 12 months the Spearman analysis results were coefficient p = 0.08 and regression p = 0.0199. For the comparison of VA and MFERG area 2 at 12 months, the Spearman correlation showed coefficient p = 0.26 and regression p = 0.044 (borderline statistical significant correlation of the final VA at 12 months and the MFERG values for area1 and area 2).
No statistically significant correlation was found between OCT and MFERG values preoperatively and in all postoperative examinations.
We found that there was a statistically significant difference between the >420 μm group and the ≤420 μm group in the preoperative VA (p = 0.02) but not 12 months postoperatively. Mean preoperative VA was lower at the group of >420 μm (20/115) than the ≤420 group (20/75). Final VA was similar in both groups (20/39 for the >420 μm group and 20/42 for the ≤420 group at 12 months) as demonstrated in Table 3. We also studied whether preoperative VA was a prognostic factor for postoperative VA. We compared the 2 groups with preoperative VA>20/100 and ≤20/100 with the VA at 12 months. Table 4 shows that the preoperative mean VA was 20/63 for the first group and 20/125 for the second group, while the mean values at 12 months were similar; 20/44 for the first group and 20/40 for the second group.
Table 3.
Mean preoperative and postoperative VA for the 2 groups according to OCT thickness
| Mean VA (preop) | Mean VA (at 12 m) | |
|---|---|---|
| Preop OCT > 420 μm N = 12 | 20/115 | 20/39 |
| Preop OCT ≤ 420 μm N = 8 | 20/75
p = 0.02 |
20/42
p > 0.05 |
Abbreviations: VA, visual acuity; OCT, optical coherence tomography; preop, preoperatively; m, months; N, number of patients.
Table 4.
Mean preoperative and postoperative VA for the 2 groups according to preoperative VA
| Mean VA (preop) | Mean VA (at 12m) | |
|---|---|---|
| patients with preop VA > 20/100 N = 7 | 20/63 | 20/44 |
| patients with preop VA ≤ 20/100 N = 13 | 20/125 | 20/40 |
Abbreviations: VA, visual acuity; preop, preoperatively; m, months; N, number of patients.
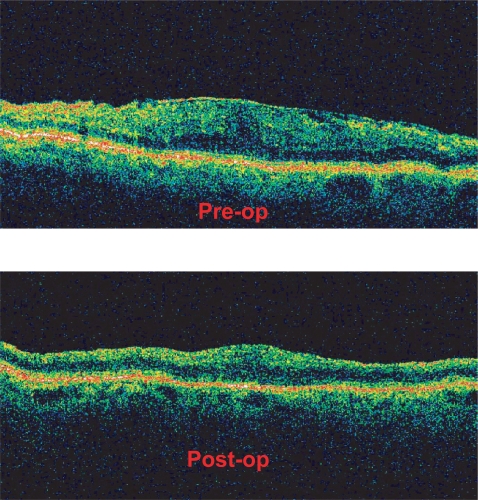
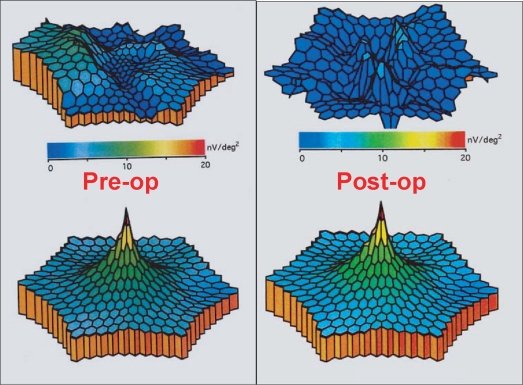
Figure 4 illustrates the preoperative (A) and postoperative OCT (B) of patient No 18, respectively. The preoperative retinal thickness of this patient was 494 μm, while the 12 months postoperative measurement was 224 μm. In Figure 5, we can see the same patients’ MFERG preoperatively (A) and 12 months postoperatively (B), where an increase of the MFERG spikes is obvious.
Figure 4.
Preoperative and postoperative OCT of patient No 18. (Top) The preoperative retinal thickness of this patient was 494 μm and the epiretinal membrane is well defined. (Bottom) The 12 months postoperative measurement was 224 μm and the OCT confirms complete epiretinal membrane removal.
Figure 5.
The same patient’s (No 18) preoperative and postoperative MFERG. (Top left) The patient’s preoperative MFERG reveals depression of the foveal and perifoveal areas. (Top right) Twelve months postoperatively MFERG shows distinct increase of the MFERG spikes. (Bottom left and right) MFERG normal reference.
Between months 3 and 6, 5/20 of our patients (25%) showed reduction or nonimprovement in VA due to progression of cataract and were all successfully operated with phacoemulsification between months 6 and 12. At 12 months VA was restored or improved in correlation with the VA at 3 months. Despite the fact that cataract formation was present postoperatively in some of our cases, statistical analysis for VA showed statistically significant improvement in all postoperative examinations. Permanent or serious complications were not noted in any of our patients during the follow-up period.
Discussion
It is estimated that almost 10% of patients with idiopathic ERMs experience progressive visual loss and 5% end up with VA of 20/200 or worse (McDonald et al 1994). After surgery, visual improvement of two or more lines of Snellen chart occurs in approximately 78% to 87% in series of patients operated on without the use of ICG dye (Machemer 1978; Margherio et al 1985; de Bustros et al 1988; Mittleman et al 1989). In one series, vision was unchanged in 9% and worse in 4% (de Bustros et al 1988). In one of the first reports of 184 eyes with idiopathic ERMs and vitrectomy (without ICG or ILM peeling), 14% patients had final VA of 20/30 or better and 44% had final VA of 20/50 or better (Margherio et al 1985). In our study, 85% of our patients achieved VA ≥20/50 and 25% achieved VA≥20/32, 70% had ≥2 Snellen lines improvement, 15% had small improvement of 1 line, 10% had the same preoperative and final VA, and 5% had worse VA at the end of follow-up.
After the first report on autopsy eyes by Burk and colleagues (2000) that ICG distinctly stains the nearly invisible retinal ILM and facilitates ILM peeling by providing a stark contrast between the stained ILM and the unstained retina, Kusaka and colleagues (2001) reported that removal of ERM and ILM can be facilitated by using ICG during vitrectomy because ILM is stained green, but ERM remains unstained and because they are clearly identified, they can be completely removed. Many studies showed that ICG was very helpful for both macular hole and epimacular membrane surgery because of the visualization of the ILM and the uncolored central area of the ERM. Ullern and colleagues (2002) used ICG, even after the removal of the membrane with a second injection, to check for possible persistence of the ILM. The first studies insisted in 2 aspects: the staining pattern of the ILM in contrast with the ERM, and the facilitation of their removal because of this staining pattern. Later articles (Sorcinelli 2003) focused on VA improvement and the anatomical success of the removal, without any postoperative recurrence of the ERM.
The Haritoglou and colleagues (2003) study showed that patients operated with ICG dye for staining showed no difference in median BCVA before and after surgery (20/63). Improvement in vision was noted only in 55% of patients, decrease in 35%, and large visual field defects in 7/20 patients, all these were attributed to the use of ICG intraoperatively. The same conclusion about the presence of visual field defects postoperatively was found in another study by Uemura and colleagues (2003). Four of 7 (57%) patients with ICG-assisted ERM peeling had peripheral visual field defects postoperatively, while none of 9 patients who underwent vitrectomy for ERM, without the use of ICG, had visual field defects. Various other articles support the idea of ILM peeling with very good results.
Park and colleagues (2003) found 100% VA improvement and 100% anatomical success after ILM peeling. Hasegawa and colleagues (2004) found VA improvement after similar ERM surgery of >2 lines at 49%, 57%, and 65% of their patients at 3, 6, and 12 months postoperatively. They also noticed that VA stabilizes throughout the first postoperative year. Our improvement of >2 Snellen lines for the same postoperative months was 40%, 65%, and 70% (and 30% at the first month) which seems clinically similar. In another article by Kwok and colleagues (2004a), the authors support the use of ICG in ERM having a mean improvement of BCVA of 3.3 lines and 14/18 patients (77.8%) with 2 or more lines improvement in a group of 13 primary and 5 secondary ERM at a mean follow up of 19.3 months. In our study, 70% of our patients showed 2 or more lines improvement at the end of follow up (12 months) and mean increase of BCVA was 3.7 lines, which seems also clinically comparable.
Koestinger and Bovey (2005) suggest that functional results, based on VA, with and without use of ICG are similar. Hillenkamp and colleagues (2005) compared BCVA and OCT measurements in a retrospective study for ERM removal with and without the use of ICG. The authors found similar results in VA improvement and anatomical results (morphology of the retina, residual ERM, or recurrence of the ERM) in the two groups. Kwok and colleagues (2004b) support its use in certain cases in order to improve visualization and facilitate the operation, especially in early ERM.
In our study we used MFERG as a more sensitive and objective examination than VA or visual fields, for evaluation of our functional results. The fact that MFERG values were the last to show increase in comparison with VA or OCT improvement may be explained by the fact that after ERM surgery the retina remains still with residual pathology. Foveal retinal thickness, despite improvement, only in 4/20 patients returned near to normal (≤210 μm) in our study and also only 4/20 patients achieved near to normal VA ≥ 20/25 at 12 months. Analysis also showed that postoperative VA did not correlate with retinal thickness measured by OCT. This means that better VA doesn’t correlate with thinner retina at the end of follow up. It also indicates that anatomical recovery is not the only decisive factor for functional improvement in patients with ERM. MFERG may depict this remaining dysfunction even more sensitively. Niwa and colleagues (2003) came to a similar conclusion when examining with focal macular (but not multifocal) electroretinograms of 15 degrees stimulus in eyes with unilateral ERM before and after surgery in comparison with fellow eyes. In the study of Moschos and colleagues (2001) with the use of MFERG, the writers found 100% improvement of area 1 and area 2 values in 5/5 patients operated on for ERM after 6 months. In our study 80% of the patients had improvement in 6 months and 100% at 12 months. Most recently, Lai and colleagues (2006) also assessed macular function with MFERG after ICG-assisted ERM vitrectomy with a follow up of 6 months. In the group with the same ICG concentration with that of our study (0.5 mg/ml), examination showed no significant change in MFERG responses at 3 and 6 months, despite significant increase of VA from a mean value of 20/70 preoperatively to 20/30 at 6 months. At a higher ICG concentration (1.25 mg/ml), MFERG values showed reduction at 3 months, and no difference at 6 months, leading the authors to suggest possible transient retinal dysfunction at the high concentration of ICG due to the dye. It is interesting that our results are similar for the first 6 months and show statistically significant improvement of MFERG values at 12 months for the 0.5 mg/ml concentration we used in all our patients. It is also interesting to find that the improved MFERG values at 12 months correlate with VA at 12 months, which means that subjective and objective functional results correlate at the end of the follow-up.
In an Ishida and colleagues (2004) study, mean retinal thickness measured by OCT reduced from 409.9 μm preoperatively to 304.6 at 6 months and to 274.3 at 1 year. Preoperative thickness ranged from 205–575 μm. In our study mean retinal thickness for the same postoperative periods was found to reduce from 472.3 μm preoperatively (326–705 μm) to 280.5 μm at 6 months and 249.2 μm at 12 months. It is interesting to notice that the rapid decrease of the foveal thickness, which we observed at the first month (362 μm), was already noted by these authors in the first week (347.7 μm). Persistent decrease of macular thickness postoperatively was also found in the Niwa and colleagues (2002) study. In our study, OCT measurements for retinal thickness and VA had a negative correlation only preoperatively, but not at any postoperative examination, despite the fact that both measurements had significant improvement postoperatively. This indicates that postoperative functional recovery is a more complicated mechanism, not explained only by postoperative retinal thickness measured by OCT.
In our study we did not find any correlation between OCT values and MFERG measurements, which fortifies our previous conclusion. To our knowledge this is the first time to correlate MFERG values with foveal thickness measured by OCT or VA in ERM surgery. Since electroretinography depicts mainly the function of the rods and cons, this might indicate that the remaining functional pathology after ERM peeling is focused on the photoreceptors. It is also important that anatomical and functional recovery of all parameters, measured in our study, continued throughout the first postoperative year. In conclusion, according to our observations, improvement of visual acuity after surgery is based not only on the anatomical improvement but also on the slower functional recovery of the photoreceptors.
Progressive nuclear cataract occurs in approximately 50%–75% of patients postoperatively and typically results in the need for cataract surgery within 1 to 3 years of vitrectomy. In our study 25% (5/20) of our patients were successfully operated within the first year.
Reviews of surgical series have shown inconsistent data regarding preoperative prognostic factors and ultimate visual improvement (de Bustros et al 1988). In an early study by Rice and colleagues (1986) of 264 cases of various etiologies, eyes with poor preoperative vision tended to improve more frequently and to greater extent than did eyes with better preoperative vision. However eyes with lower preoperative VA usually had a lower final vision than eyes with better preoperative VA. In our study eyes with lower preoperative VA improved more than eyes with better preoperative VA, but at the end of follow up the two groups had similar VA.
In this study, additionally to the preoperative vision, we also considered the preoperative retinal thickness as a possible prognostic factor. We compared the two groups of very increased preoperative retinal thickness (>420 μm) and smaller retinal thickness (≤420 μm) to the postoperative VA results. We found that patients with very increased values in preoperative OCT measurements had lower mean VA preoperatively, but they had similar VA at 12 months, compared with the patients with smaller preoperative retinal thickness. However the patients with increased values in preoperative OCT measurements finally gained more Snellen lines postoperatively compared with the patients of the smaller preoperative group, since they started up with statistically significant lower VA.
In conclusion, in our series of eyes with successful ERM surgery BCVA, MFERG, and OCT values statistically improved twelve months postoperatively. OCT retinal thickness measurements and VA gradually improved throughout the first postoperative year, with a statistically significant improvement at all postoperative examinations, while MFERG functional results showed statistically significant improvement at 12 months. For this reason, we believe that ICG-assisted ERM and ILM removal was a safe technique with good functional results in our series.
References
- Burk SE, Da Mata AP, Snyder ME, et al. Indocyanine green-assisted peeling of the retinal internal limiting membrane. Ophthalmology. 2000;107:2010–14. doi: 10.1016/s0161-6420(00)00375-4. [DOI] [PubMed] [Google Scholar]
- de Bustros S, Thompson JT, Michels RG, et al. Vitrectomy for idiopathic epiretinal membranes causing macular pucker. Br J Ophthalmol. 1988;72:692–5. doi: 10.1136/bjo.72.9.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haritoglou C, Gandorfer A, Gass CA, et al. Indocyanine green-assisted peeling of the internal limiting membrane in macular hole surgery affects visual outcame: a clinicopathologic correlation. Am J Ophthalmol. 2002;134:836–41. doi: 10.1016/s0002-9394(02)01816-0. [DOI] [PubMed] [Google Scholar]
- Haritoglou C, Gandorfer A, Gass CA, et al. The effect of indocyanine-green on functional outcome of macular pucker surgery. Am J Ophthalmol. 2003;135:328–37. doi: 10.1016/s0002-9394(02)01969-4. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Emi K, Ikeda T, et al. Long-term prognosis of internal limiting membrane peeling for idiopathic epiretinal membrane. Nippon Ganka Gakkai Zasshi. 2004;108:150–6. [PubMed] [Google Scholar]
- Hillenkamp J, Saikia P, Gora F, et al. Macular function and morphology after peeling of idiopathic epiretinal membrane with and without the assistance of indocyanine green. Br J Ophthalmol. 2005;89:437–43. doi: 10.1136/bjo.2004.051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa H, Jalkh AE, Takahas HIM, et al. Role of the vitreous in idiopathic preretinal macular fibrosis. Am J Ophthalmol. 1986;101:166–9. doi: 10.1016/0002-9394(86)90589-1. [DOI] [PubMed] [Google Scholar]
- Ishida M, Takeuchi S, Nakamura M, et al. The surgical outcome of vitrectomy for idiopathic epiretinal membranes and foveal thickness before and after surgery. Nippon Ganka Gakkai Zasshi. 2004;108:18–22. [PubMed] [Google Scholar]
- Iwanoff A. Beitrage zur normalen und pathologischen anatomy des auges. Graefes Arch Clin Exp Ophthalmol. 1865;11:135. [Google Scholar]
- Koestinger A, Bovey EH. Visual acuity after vitrectomy and epiretinal membrane peeling with or without premacular indocyanine green injection. Eur J Ophthalmol. 2005;15:795–9. doi: 10.1177/112067210501500622. [DOI] [PubMed] [Google Scholar]
- Kusaka S, Hayashi N, Ohji M, et al. Indocyanine green facilitates removal of epiretinal and internal limiting membranes in myopic eyes with retinal detachment. Am J Ophthalmol. 2001;131:388–90. doi: 10.1016/s0002-9394(00)00848-5. [DOI] [PubMed] [Google Scholar]
- Kwok AK, Lai TY, Li WW, et al. Indocyanine green assisted internal limiting membrane removal in epiretinal membrane surgery. Am J Ophthalmol. 2004a;138:194–9. doi: 10.1016/j.ajo.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Kwok AK, Lai TY, Li WW, et al. Trypan blue- and indocyanine green-assisted epiretinal membrane surgery: clinical and histopathological studies. Eye. 2004b;18:882–8. doi: 10.1038/sj.eye.6701359. [DOI] [PubMed] [Google Scholar]
- Lai TY, Kwok AK, Au AW, et al. Assessment of macular function by multifocal electroretinography following epiretinal membrane surgery with indocyanine green-assisted internal limiting membrane peeling. Graefes Arch Clin Exp Ophthalmol. 2007;245:148–54. doi: 10.1007/s00417-006-0352-0. [DOI] [PubMed] [Google Scholar]
- Machemer R. Die chirurgische entfernung von epiretina en makulamembranen (macular puckers) Klin Monatsbl Augenheilkd. 1978;173:36–42. [PubMed] [Google Scholar]
- Margherio RR, Cox MS, Jr, Trese MT, et al. Removal of epimacular membranes. Ophthalmology. 1985;92:1075–83. doi: 10.1016/s0161-6420(85)33902-7. [DOI] [PubMed] [Google Scholar]
- McDonald HR, Shatz H, Johnson RN. Introduction to epiretinal membranes. In: Ryan SJ, editor. Retina. 2nd ed. St. Louis: Mosby; 1994. pp. 1819–23. [Google Scholar]
- Mittleman D, Green WR, Michels RG, et al. Clinicopathologic correlation of an eye after surgical removal of an epiretinal membrane. Retina. 1989;9:143–7. doi: 10.1097/00006982-198909020-00015. [DOI] [PubMed] [Google Scholar]
- Moschos M, Apostolopoulos M, Ladas J, et al. Assessment of macular function by multifocal electroretinogram before and after epimacular membrane surgery. Retina. 2001;21:590–5. doi: 10.1097/00006982-200112000-00005. [DOI] [PubMed] [Google Scholar]
- Niwa T, Terasaki H, Kondo M, et al. Function and morphology of macula before and after removal of idiopathic epiretinal membrane. Invest Ophthalmol Vis Sci. 2003;44:1652–6. doi: 10.1167/iovs.02-0404. [DOI] [PubMed] [Google Scholar]
- Park DW, Dugel PU, Garda J, et al. Macular pucker removal with and without internal limiting membrane peeling: pilot study. Ophthalmology. 2003;110:62–4. doi: 10.1016/s0161-6420(02)01440-9. [DOI] [PubMed] [Google Scholar]
- Rice TA, de Bustros S, Michels RG, et al. Prognostic factors in vitrectomy for epiretinal membranes of the macula. Ophthalmology. 1986;93:602–10. doi: 10.1016/s0161-6420(86)33689-3. [DOI] [PubMed] [Google Scholar]
- Sorcinelli R. Surgical management of epiretinal membrane with indocyanine-green-assisted peeling. Ophthalmologica. 2003;217:107–10. doi: 10.1159/000068556. [DOI] [PubMed] [Google Scholar]
- Uemura A, Kanda S, Sakamoto Y, et al. Visual field defects after uneventful vitrectomy for epiretinal membrane with indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2003;136:252–7. doi: 10.1016/s0002-9394(03)00157-0. [DOI] [PubMed] [Google Scholar]
- Ullern M, Roman S, Dhalluin JF, et al. Contribution of intravitreal infracyanine green to macular hole and epimacular membrane surgery: preliminary study. J Fr Ophtalmol. 2002;25:915–20. [PubMed] [Google Scholar]