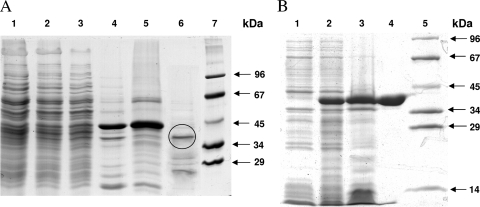
FIG. 2.
SDS-PAGE (12.5%) of fractions obtained during purification of native and recombinant acryloyl-CoA reductase. Proteins were stained with Coomassie blue. (A) Enzyme fractions during purification of the native enzyme from M. sedula. Lane 1, cell extract of autotrophically grown cells (20 μg); lane 2, after heat precipitation (20 μg); lane 3, after ultracentrifugation (20 μg); lane 4, after Q-Sepharose chromatography (20 μg); lane 5, after carboxymethylcellulose chromatography (20 μg); lane 6, after phenyl-Sepharose chromatography (10 μg; after an additional purification step using a Resource S column the marked band was cut out and sequenced); lane 7, molecular mass standard proteins. (B) Heterologous expression of the acryloyl-CoA reductase gene from S. tokodaii in E. coli Rosetta 2 (DE3). Lane 1, whole cells before induction; lane 2, whole cells after 3 h of induced growth; lane 3, cell extract after heat precipitation (20 μg); lane 4, purified recombinant acryloyl-CoA reductase after Ni2+ affinity column (20 μg); lane 5, molecular mass standard proteins.