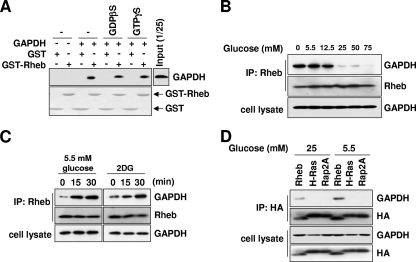
FIG. 2.
GAPDH interacts with Rheb, and the interaction is regulated by glycolytic flux. (A) The interaction of Rheb with GAPDH does not depend on its nucleotide binding status. GST or GST-Rheb was charged with 100 μM GDPβS or 100 μM GTPγS. Each species of GST or GST-Rheb was incubated with purified rabbit muscle GAPDH. After GST pulldown assays were performed, bound GAPDH was analyzed by immunoblotting with anti-GAPDH antibodies (upper panel). The amount of GST-Rheb used was shown by Ponceau S staining (lower panel). (B) Interaction between Rheb and GAPDH is regulated by extracellular glucose availability. HEK293 cells were incubated in media containing the indicated concentrations of glucose for 30 min. Lysates prepared from these cells were immunoprecipitated with anti-Rheb antibodies. GAPDH bound to Rheb was analyzed by anti-GAPDH immunoblotting. (C) The inhibition of glycolytic flux induces Rheb-GAPDH interaction. HEK293 cells were treated with 25 mM 2DG or incubated in 5.5 mM glucose medium for the indicated times. Lysates prepared from these cells were immunoprecipitated with anti-Rheb antibodies. GAPDH bound to Rheb was analyzed by anti-GAPDH immunoblotting. (D) GAPDH specifically interacts with Rheb but not other Ras GTPases. HEK293 cells were transfected with wild-type HA-Rheb, HA-H-Ras, or HA-Rap2A and incubated in media containing the indicated concentration of glucose for 30 min. Lysates prepared from these cells were immunoprecipitated with anti-HA antibodies. GAPDH bound to Rheb was analyzed by anti-GAPDH immunoblotting.