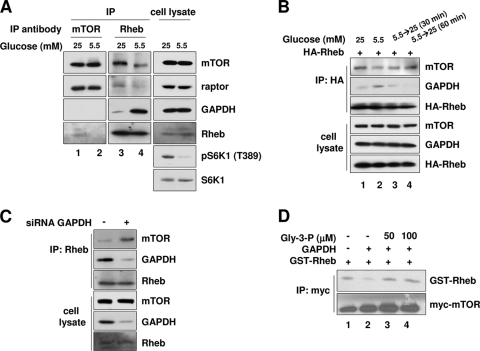
FIG. 5.
GAPDH causes Rheb to dissociate from mTORC1 under low-glucose conditions. (A) Coimmunoprecipitation of endogenous proteins. HEK293 cells were incubated with media with the indicated concentrations of glucose for 30 min and then immunoprecipitated with either anti-mTOR (lanes 1 and 2) or anti-Rheb (lanes 3 and 4) antibodies. Immunoprecipitates were blotted with the indicated antibodies for mTOR, raptor, Rheb, GAPDH, pS6K1, and S6K1. (B) Glucose depletion-induced Rheb-GAPDH binding reduces Rheb-mTOR association. HEK293 cells transfected with HA-Rheb were incubated in 5.5 mM glucose medium for 30 min and then incubated with 25 mM glucose medium for the indicated times. Cell lysates were immunoprecipitated with anti-HA antibodies and then immunoblotted with anti-mTOR and anti-GAPDH antibodies. (C) GAPDH knockdown increases Rheb-mTOR association. HEK293 cells were transfected with GAPDH_#1 or luciferase siRNA. Cell lysates were immunoprecipitated with Rheb antibodies. Immunoprecipitates were blotted with the indicated antibodies for mTOR, GAPDH, and Rheb. (D) GAPDH inhibits the association between Rheb and mTORC1 in vitro. The Myc-tagged mTOR immunocomplexes were prepared from HEK293 cells and incubated with soluble GST-Rheb in the presence or absence of purified rabbit muscle GAPDH, with or without Gly-3-P. GST-Rheb bound to the mTOR complex was analyzed by anti-Rheb immunoblotting. Amounts of Myc-mTOR immunocomplexes used are shown by anti-mTOR immunoblotting.