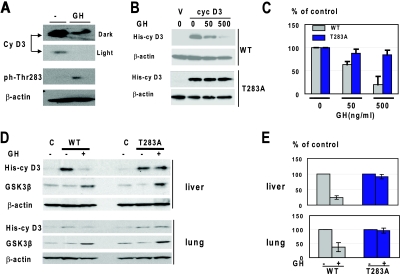
FIG. 6.
GH-mediated degradation of cyclin D3 in cultured cells and in mouse tissues requires Thr283. (A) Treatment of Hep3B2 cells with GH phosphorylates cyclin D3 at Thr283. Hep3B2 cells were treated with GH (50 ng/ml), and protein extracts were examined by Western blotting with antibodies to total cyclin D3 and to the Thr283-ph isoform of cyclin D3. Dark and light exposures of total cyclin D3 are shown. The membrane was reprobed with antibodies to β-actin. (B) Mutation of Thr283 to Ala blocks the ability of GH to reduce cyclin D3 in Hep3B2 cells. Hep3B2 cells were transfected with vectors expressing WT His-cyclin D3 and Thr283A-His-cyclin D3. The cells were treated with increasing concentrations of GH as shown. Total cell lysates were analyzed by Western blotting with antibody against the His tag. The filters were reprobed with antibodies to β-actin. (C) Protein levels of cyclin D3 are shown as a summary of three independent experiments. Error bars indicate standard deviations. (D) GH fails to reduce levels of the Thr283A mutant cyclin D3 in livers and lungs of old mice. WT His-cyclin D3 and T283A-His-cyclin D3 were delivered into old mice and into animals pretreated with GH. Nuclear extracts were examined by Western blotting using antibodies against the His tag and GSK3β. (E) Levels of cyclin D3 were calculated as ratios to β-actin. The bar graphs show summaries of two independent experiments with four controls and four cyclin D3-injected mice.