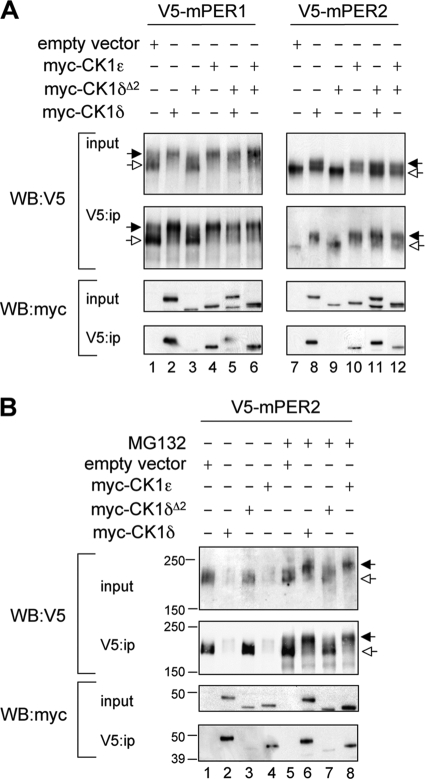
FIG. 2.
CK1δΔ2 is a functional null. (A) Assessment of the functional activity of CK1δΔ2 coexpressed with mPER proteins. Myc-tagged casein kinases (CK1δ, CK1ɛ, and CK1δΔ2) and V5-tagged mPER proteins were coexpressed in HEK293 cells (input), and protein complexes were immunoprecipitated with an antibody to V5 (V5:ip). Kinase activity is reflected by an upward mobility shift of mPER proteins (indicated by the filled arrow; seen in the V5 Western blots in lanes 2, 4, 5, 6, 8, and 10, but not in lanes 1, 3, 7, and 9). Hypophosphorylated mPER proteins are indicated by the open arrows. The lower panel shows that myc-tagged CK1δ (lanes 2 and 8) and CK1ɛ (lanes 4 and 10) coprecipitate with the PER proteins, while CK1δΔ2 does not (lanes 3 and 9). Notably, expression of CK1δΔ2 does not interfere with the mobility shift or coprecipitation activities of CK1δ and CK1ɛ (lanes 5, 6, 11, and 12). The results shown are representative of four independent experiments. (B) CK1-mediated proteasomal degradation of mPER2. Cellular coexpression studies similar to those described above were conducted in HEK293 cells, except that a higher concentration of kinase plasmid was used to further promote PER protein degradation. Studies were conducted in the absence (left) or presence (right) of MG132 (10 μM). The black and white arrows indicate hyper- and hypophosphorylated mPER2, respectively. CK1δ and CK1ɛ promote degradation of mPER2 (lanes 2 and 4), while CK1δΔ2 does not (lane 3). In the presence of MG132, hyperphosphorylated mPER2 accumulates rather than being degraded. CK1δ and CK1ɛ promote accumulation of hyperphosphorylated mPER2 (lanes 5 and 7), while CK1δΔ2 does not (lane 6). The results shown are representative of three independent experiments.