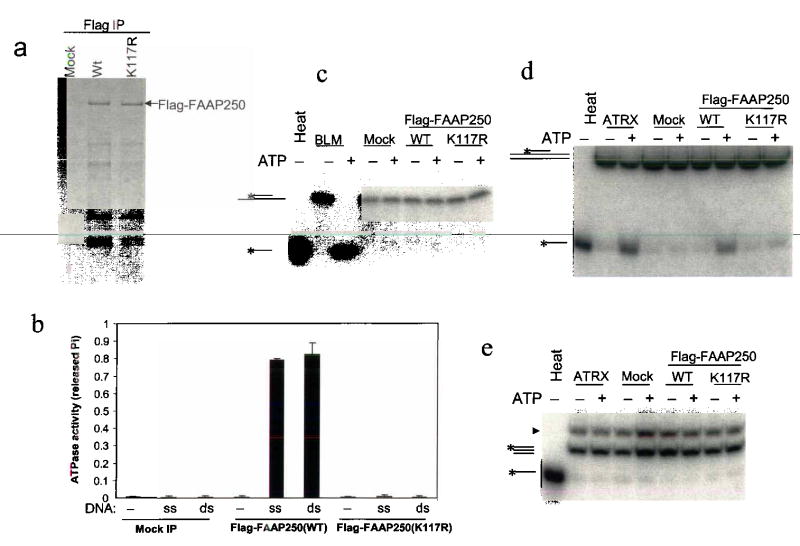
Fig. 4. FAAP250 has an ATP-dependent DNA translocase activity.
(a) A silver-stained SDS-gel showing the immunoisolated Flag-FAAP250 of wildtype and K117R mutant from HEK293-derived cells stably expressing these proteins. Polypeptides immunoisolated from HEK293 cells that do not express the Flag-tagged proteins are shown as a control (Mock). (b) A histogram shows that Flag-FAAP250 has DNA-stimulated ATPase activity, whereas a point mutation within the conserved helicase motif I (K117R) inactivates such activity. The double-stranded (ds) and single-stranded (ss) DNA are indicated. (c) An autoradiograph showing that Flag-FAAP250 exihibits no detectable helicase activity in a double-strand displacement assay. BLM helicase-associated complexes (BLM)4 were used as a positive control. The DNA substrate has 13-nucleotide single-stranded tails on both sides of a 20-bp double-stranded region, so that it should be able to detect activity of helicases of either 5′ to 3′ or 3′ to 5′ directions. (d) Autoradiograph showing that Flag-FAAP250 displays a triple-helix unwinding activity, whereas its ATPase mutant (K117R) lacks such activity. A chromatin-remodeling complex, ATRX complex30, was used as a positive control. The substrate consists of a 40-base homopyrimidine oligonucleotide located centrally on a 190 bp double-stranded DNA23. (e) Autoradiograph showing that Flag-FAAP250 does not displace a blunt triple helix. The substrate is the same as in (d), except that the duplex DNA is the same size (40 bp) as the homopyrimidine oligonucleotide, to create the blunt triplex. The band with faster mobility is from the designed blunt triplex substrate. The band with slower mobility (marked with an arrowhead) is from the precursor of the substrate.