Abstract
Objective: To demonstrate the validity and reliability of volumetric quantitative computed tomography (vQCT) with multi-slice computed tomography (MSCT) and dual energy X-ray absorptiometry (DXA) for hip bone mineral density (BMD) measurements, and to compare the differences between the two techniques in discriminating postmenopausal women with osteoporosis-related vertebral fractures from those without. Methods: Ninety subjects were enrolled and divided into three groups based on the BMD values of the lumbar spine and/or the femoral neck by DXA. Groups 1 and 2 consisted of postmenopausal women with BMD changes <−2SD, with and without radiographically confirmed vertebral fracture (n=11 and 33, respectively). Group 3 comprised normal controls with BMD changes ≥−1SD (n=46). Post-MSCT (GE, LightSpeed16) scan reconstructed images of the abdominal-pelvic region, 1.25 mm thick per slice, were processed by OsteoCAD software to calculate the following parameters: volumetric BMD values of trabecular bone (TRAB), cortical bone (CORT), and integral bone (INTGL) of the left femoral neck, femoral neck axis length (NAL), and minimum cross-section area (mCSA). DXA BMD measurements of the lumbar spine (AP-SPINE) and the left femoral neck (NECK) also were performed for each subject. Results: The values of all seven parameters were significantly lower in subjects of Groups 1 and 2 than in normal postmenopausal women (P<0.05, respectively). Comparing Groups 1 and 2, 3D-TRAB and 3D-INTGL were significantly lower in postmenopausal women with vertebral fracture(s) [(109.8±9.61) and (243.3±33.0) mg/cm3, respectively] than in those without [(148.9±7.47) and (285.4±17.8) mg/cm3, respectively] (P<0.05, respectively), but no significant differences were evident in AP-SPINE or NECK BMD. Conclusion: the femoral neck-derived volumetric BMD parameters using vQCT appeared better than the DXA-derived ones in discriminating osteoporotic postmenopausal women with vertebral fractures from those without. vQCT might be useful to evaluate the effect of osteoporotic vertebral fracture status on changes in bone mass in the femoral neck.
Keywords: Osteoporosis, Bone mineral density (BMD), Volumetric QCT, Hip fracture, Postmenopausal women
INTRODUCTION
Hip fracture is generally considered the most serious immediate outcome of osteoporosis, and is becoming increasingly frequent internationally at a rate of approximately 1% to 3% per year (Cummings and Melton, 2002). Across most regions of the world, hip fracture incidence rates in both women and men have been shown to increase exponentially with age. This dramatic rise in fracture risk mainly results from an age-related decrease in bone mineral density (BMD) at the proximal femur (Melton and Cooper, 2001). In addition to dual-energy X-ray absorptiometry (DXA), quantitative computed tomography (QCT) is a traditional method of bone mineral content evaluation. QCT has advantages over DXA, primarily in its ability to independently assess trabecular and cortical BMDs and generate geometric information that is not attainable with projectional techniques (Genant et al., 1996).
Recently, volumetric QCT (vQCT) techniques have been introduced gradually for use in clinical trials. They also may independently assess the influences of the size, shape, and structure of bone parameters that are regarded as components of bone quality, rather than bone quantity on changes in bone strength (Lian et al., 2005; Huber et al., 2008), especially in the proximal femur where the anatomic features and architecture are complex. To the best of our knowledge, no previously-published study has compared the results of volumetric BMD measurement by multi-slice CT (MSCT) in the femoral neck of postmenopausal women with and without osteoporosis-related vertebral fractures. To demonstrate differences in the vBMD of the femoral neck between postmenopausal osteoporotic women with and without vertebral fractures, we used an MSCT scanner and the vQCT technique to conduct vBMD measurements of the femoral neck, and then evaluated the effect of vertebral fracture status on various parameters indicating the changes in bone strength of the femoral neck.
MATERIALS AND METHODS
Study population
Participants in this study included 90 in-patient postmenopausal women [mean age (59.8±5.9) years], who consecutively underwent abdominal-pelvic MSCT scanning (using a phantom, Imaging Analysis Co., USA) at General Hospital of Tianjin Medical University from February 2006 to May 2008. Study eligibility required that the post-menopausal women participants meet the following inclusion/exclusion criteria: (1) age ≥50 years old, (2) no metastatic or other metabolic bone disease, (3) no history of malignant bone marrow infiltration, (4) no prior radiation therapy, and (5) willing and able to provide informed written consent. The local Institutional Review Board (IRB) approved this study protocol, and informed consent was obtained from each participant.
All subjects underwent DXA BMD measurements of the proximal femur and lumbar spine (by anterior-posterior (AP) projection), from which a diagnosis of osteoporosis was either established or rejected. Subsequently, subjects were divided into three groups, based upon the BMD values of their lumbar spines by DXA (Wu et al., 2005). Group 1 consisted of women with BMD changes showing more than 2 standard deviations below the mean (BMD changes <−2SD), who had convincing radiographic evidence of one or more non-traumatic vertebral fractures (n=11). Group 2 consisted of women with BMD changes <−2SD who had no evidence of any non-traumatic vertebral fractures (n=33). Group 3 was composed of controls with BMD changes ≥−1SD (n=46) (Table 1). The 44 women in Groups 1 and 2 also underwent thoracolumbar radiographic examination, with vertebral deformities assessed using the Genant grading scale (Genant et al., 1993).
Table 1.
Demographic and clinical characteristics of the subjects
| n | Age (year) | Height (cm) | Weight (kg) | |
| All subjects | 90 | 59.8±5.9 | 158.7±5.8 | 61.8±8.6 |
| Group 1 | 11 | 63.2±5.4 | 155.4±5.4 | 57.3±4.8 |
| Group 2 | 33 | 62.9±5.5 | 159.2±5.6 | 61.8±9.8 |
| Group 3 | 46 | 56.8±4.6 | 159.0±5.5 | 64.1±6.1 |
All data expressed as mean±SD
BMD and geometric measurements of the proximal femur
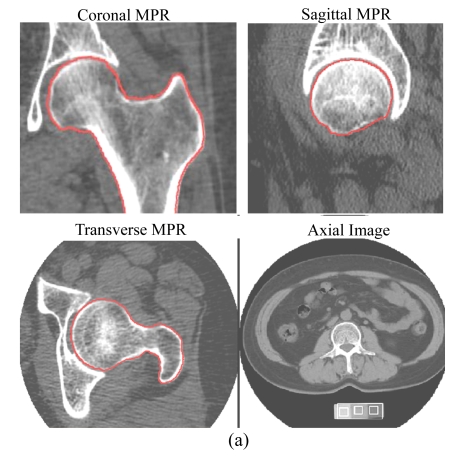
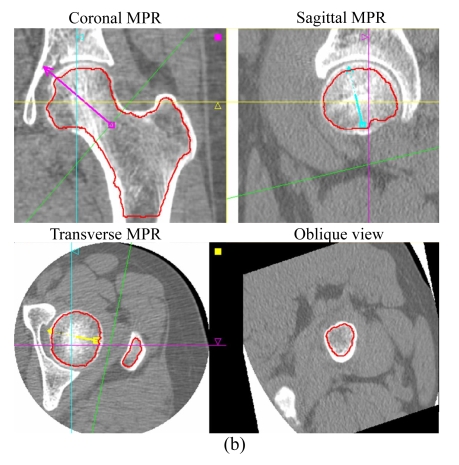
The image dataset from MSCT (LightSpeed16, GE Healthcare, Milwaukee, USA) scans (helical mode, pitch 1.375:1, 120 kV, 240 mA·s, 10 mm beam width, 16 channels) of the proximal femur of all subjects, with a phantom (Image Analysis Company, Columbia, KY, USA), was used for reconstruction into images, 1.25 mm thick per slice, of the left proximal femur; and then these multiplanar reformation (MPR) images were transferred and processed by means of OsteoCAD software (Neusoft Medical System Co., Ltd., Shenyang, China) to generate measurements of volumetric BMD (mg/cm3) of the femoral neck. These included measurements of trabecular bone (TRAB), cortical bone (CORT) and integral bone (INTGL) using a previously-published method (Kang et al., 2005) incorporating an anatomic coordinate system (semi-automatic volumetric segmentation) (Fig.1). Using the same software for hip geometric measurements, neck axis length (NAL), measured between the lateral edge of the greater trochanter and the medial edge of the femoral head, and the minimum cross-section area (mCSA), the regions across the narrowest plane of the femoral neck, were calculated automatically.
Fig.1.
3D segmentations of MSCT images of the left proximal femur
(a) Coronal, sagittal, and transverse MPRs showing the periosteal surface recognized (in red color), and an axial image with a phantom; (b) The endosteal surface is shown in red in coronal, sagittal, and transverse MPRs, and the position of the oblique view indicates the plane of the femoral neck with mCSA
Precision was defined as the root-mean square of the coefficient of variation (CV) calculated from the scan pairs (Glüer et al., 1995) and used to evaluate the reproducibility of each vBMD and geometric measurement by quantitative MSCT. The inter-observer reproducibility of the vQCT assessments of hip vBMD and geometric measurement was estimated by repetitive scanning of a random sample of 10 subjects, via repositioning of patients and the phantom within 2 weeks, and blinded readings were performed by 2 radiologists independently. To study intra-observer reliability, 1 radiologist reanalyzed these images 2 months after the initial readings.
DXA BMD measurements were conducted using the Lunar DPX-L (GE-Lunar, Wisconsin, USA) device, at both the lumbar spine (AP projection) and left hip of each subject, with AP-SPINE and NECK (femoral neck) as the parameters of interest. Image acquisition and analysis were performed as recommended by the manufacturer. For AP-SPINE, the average value was acquired with L2~L4, among which any vertebra would be excluded when suffered from fracture. AP-SPINE and/or NECK were used in the evaluation of osteoporosis status. In this way, seven parameters were generated for comparison between the three study groups: (1) TRAB, (2) CORT, (3) INTGL, (4) NAL, (5) mCSA, (6) AP-SPINE, and (7) NECK.
Statistical analysis
Group mean and standard deviation (SD) were calculated for each of the several above-listed parameters. Inter-group comparisons for all the seven parameters were conducted using analysis of variance (ANOVA) and analysis of covariance (ANCOVA) with adjusting for age alone and adjusting for age, height, and weight together. In addition, parameters were compared with respect to both inter-observer and intra-observer reliabilities. A 5% significance level was applied for all tests (P<0.05), and all tests were two-tailed. All data were analyzed using the Windows 11.5 version of SPSS (SPSS Inc., Chicago, Illinois, USA).
RESULTS
Intra- and inter-observer precision errors in the analysis of BMD for trabecular, cortical, and integral bones at the femoral neck were 1.02% and 0.98%, 1.08% and 1.11%, and 1.07% and 1.04%, respectively. Intra- and inter-observer precision errors for NAL and mCSA at the neck were 0.16% and 0.20%, and 0.19% and 0.31%, respectively.
For all the volumetric BMDs and geometric indices, there were statistically significant differences between the two osteoporosis groups and the normal control, as well as between the two osteoporotic groups (F=16.151~108.713, P<0.01), even after adjusting for age, height and weight (F=7.080~51.566, P<0.01) (Table 2).
Table 2.
BMD values for seven parameters measured by MSCT and DXA in the three groups of postmenopausal women
| Measure | BMD value |
F | P | F* | P* | ||
| Osteoporosis |
Normal control |
||||||
| Group 1 (n=11) | Group 2 (n=33) | Group 3 (n=46) | |||||
| TRAB (mg/cm3) | 109.8±9.61†‡ | 148.9±7.47§ | 210.5±23.7 | 108.713 | <0.01 | 35.328 | <0.01 |
| CORT (mg/cm3) | 471.4±20.4‡ | 485.1±34.6§ | 538.5±40.6 | 27.146 | <0.01 | 7.080 | <0.01 |
| INTGL (mg/cm3) | 243.3±33.0†‡ | 285.4±17.8§ | 347.1±28.1 | 103.445 | <0.01 | 19.432 | <0.01 |
| NAL (mm) | 90.1±3.88‡ | 89.8±3.66§ | 79.7±3.62 | 16.151 | <0.01 | 11.708 | <0.01 |
| mCSA (mm2) | 544.9±54.3‡ | 563.2±60.8§ | 634.7±67.4 | 86.010 | <0.01 | 51.566 | <0.01 |
| AP-SPINE (g/cm2) | 0.89±0.31‡ | 0.90±0.10§ | 1.18±0.16 | 54.073 | <0.01 | 19.451 | <0.01 |
| NECK (g/cm2) | 0.67±0.05‡ | 0.72±0.05§ | 0.91±0.08 | 109.574 | <0.01 | 21.718 | <0.01 |
BMD values are expressed as mean±SD. Comparison of values:
represents significant difference of vertebral fractured group vs osteoporotic group;
represents significant difference of vertebral fractured group vs normal group;
represents significant difference of osteoporotic group vs normal group.
F, P result from ANOVA; F *, P * result from ANCOVA after adjusting for age, height and weight. P<0.05 was used as standard level of significance
Among the three volumetric BMD indices, for all but the CORT index, the values of 3D-TRAB and 3D-INTGL [(109.8±9.61) and (243.3±33.0) mg/cm3, respectively] in the osteoporotic fracture group were significantly lower than those [(148.9±7.47) and (285.4±17.8) mg/cm3, respectively] in the osteoporotic group without fractures (P<0.001 and P=0.007, respectively). Even after adjusting for age, height and weight, TRAB and INTGL values decreased by 26.4% and 14.7%, respectively. No significant differences in NAL or mCSA were identified between these two groups.
There were significant differences in mean values for AP-SPINE and NECK by DXA measurement between Groups 1 and 3, as well as between Groups 2 and 3 [(0.89±0.31) and (1.18±0.16) g/cm2, (0.90±0.10) and (1.18±0.16) g/cm2, P<0.01, respectively]. There was no significant difference in the values for AP-SPINE or NECK between Groups 1 and 2 (Table 2).
DISCUSSION
In the present study, we observed that volumetric BMD values for the trabecular and integral regions of the femoral neck in osteoporotic vertebral fracture subjects were significantly lower than those in osteoporosis subjects without vertebral fracture, with the exception of the proximal femoral cortical volumetric BMD. However, the difference in BMD values at the femoral neck using DXA between those with and without vertebral fractures was not significant in this study. This is consistent with previous reports that have shown reduced spinal and femoral BMD values in subjects with vertebral fractures, and with a few studies that have demonstrated QCT to be better than DXA in the prediction of spinal (Lafferty and Rowland, 1996; Ito et al., 1997; Lang et al., 1999; 2002) and femoral (Lang et al., 1997; 2002) bone strength and fracture risk in postmenopausal women.
Low bone density and previous fractures are risk factors for almost all types of fracture (Cummings and Melton, 2002), and the risks of hip fractures were increased with baseline prevalent vertebral deformity, with relative risk of 2.8 (95% CI=2.3~3.4) (Black et al., 1999). In the clinical setting, it is crucial to apply non-invasive imaging modalities when evaluating osteoporosis and fracture risk among postmenopausal women, especially of the hip in patients with a history of fracture. It has been reported that QCT provides more detailed information about hip sub-regions than DXA hip measurements (Lang et al., 2002; Lane et al., 1998). With the development of MSCT, the vQCT technique has emerged as a modality that utilizes the volumetric analysis of bone of the hip and provides information ranging from bone macrostructure to estimates of bone strength. In a recent in vitro study, Bousson et al.(2006) used a 3D segmentation method and investigated the ability of vQCT to assess femoral neck fractures and predict failure load at the proximal femur, in comparison with DXA. They also compared the contribution of the two modalities to bone failure load, by assessing changes in density versus geometry, and cortical bone versus trabecular bone. Their vQCT measurements, which combined densitometric with geometric variables, explained 76% of the variance in femoral failure load, versus 69% with the DXA model.
Our study compared the effect of osteoporosis status and vertebral fracture on densitometric and geometric changes in the femoral neck in postmenopausal women, using the same automated volume of interest (VOI)-finding algorithm. We preliminarily confirmed that vQCT is valid for detecting decreases in regional BMD values of the hip in post-menopausal women with osteoporosis-related vertebral fractures, as documented by MSCT images, although hip geometry measures (like NAL and mCSA) failed to demonstrate any statistical distinction between those with and without vertebral fractures. The results of this study suggest that the use of volumetric BMD measurements by means of this anatomic coordinate system can evaluate the degree of bone mass decline in the femoral neck, and may predict hip fracture risk and provide clinical bone strength assessment when combined with geometric measurements in postmenopausal women.
In this study, we estimated the precision error of in vivo 3D BMD of the femoral neck as between 0.98% and 1.11%, and found that the geometric parameters NAL and mCSA were even more precise (with 0.16% and 0.19% intra-observer reliability, and 0.20% and 0.31% inter-observer reliability, respectively). Our findings are similar to those in the study by Kang et al.(2005), who reported intra- and inter-observer precision errors for BMD in trabecular, cortical, and integral bones at the neck as 1.20% and 1.56%, 0.81% and 0.78%, and 0.57% and 0.80%, respectively. In another in vivo study, Li et al.(2006) used registration techniques, and reported precision errors of 4.53% for neck VOI and 0.60% for trochanter VOI. Our results also suggest that the 3D segmentation method used in an anatomic coordinate system has potential usefulness in monitoring different trabecular or cortical bone responses to pharmacological interventions, even in evaluating bone strength changes. In a few clinical trials in which vQCT has been used (Black et al., 2003; 2005), this method has revealed a substantial increase in volumetric trabecular BMD of both the total hip and femoral neck, as well as in trabecular BMD of the spine in parathyroid hormone (PTH)-treated postmenopausal osteoporotic women, after 1 and 2 years of treatment. With the advent of MSCT, 3D vQCT techniques are developing to further improve precision and enhance outcome sensitivity in treatment studies.
Admittedly, the current study has limitations, such as the relatively small sample size (e.g., only 11 women with osteoporosis and a fracture) and only one site of the femoral neck assessed. Further studies need to be performed involving a larger population of postmenopausal osteoporosis patients to determine or confirm differences in 3D cortical BMD and trabecular BMD values between those with and without vertebral fractures by history, using an updated version of the anatomic coordinate system that also includes other sub-regions, like trochanter VOI and other VOIs.
In conclusion, we provide evidence that BMD measurements of the femoral neck using the 3D-vQCT technique can discriminate osteoporotic postmenopausal women with vertebral fractures from those without, and that such measurements seem to have a better capacity to achieve this end than DXA-related measures. This demonstrates the potential for evaluating the effect of osteoporosis-related vertebral fracture status on changes in bone mass at the femoral neck, and suggests that volumetric BMD might be used clinically to predict hip fracture risk in postmenopausal women with a history of osteoporosis-related vertebral fractures.
Acknowledgments
We would like to thank Dr. Yan Kang at Neusoft Medical System Co., Ltd., Shenyang, China for providing OsteoCAD software used in this study.
References
- 1.Black DM, Ardenn K, Palermo L, Person J, Cummings SR. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. J Bone Miner Res. 1999;14(5):821–828. doi: 10.1359/jbmr.1999.14.5.821. [DOI] [PubMed] [Google Scholar]
- 2.Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349(13):1207–1215. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 3.Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ, PaTH Study Investigators One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005;353(6):555–565. doi: 10.1056/NEJMoa050336. [DOI] [PubMed] [Google Scholar]
- 4.Bousson V, Le Bras A, Roqueplan F, Kang Y, Mitton D, Kolta S, Bergot C, Skalli W, Vicaut E, Kalender W, et al. Volumetric quantitative computed tomography of the proximal femur: relationships linking geometric and densitometric variables to bone strength. Role for compact bone. Osteoporos Int. 2006;17(6):855–864. doi: 10.1007/s00198-006-0074-5. [DOI] [PubMed] [Google Scholar]
- 5.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359(9319):1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 6.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semi-quantitative technique. J Bone Miner Res. 1993;8(9):1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 7.Genant HK, Engelke K, Fuerst T, Glüer CC, Grampp S, Harris ST, Jergas M, Lang T, Lu Y, Majumdar S, et al. Noninvasive assessment of bone mineral and structure: state of the art. J Bone Miner Res. 1996;11(6):707–730. doi: 10.1002/jbmr.5650110602. [DOI] [PubMed] [Google Scholar]
- 8.Glüer CC, Blake G, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporosis Int. 1995;5(4):262–270. doi: 10.1007/BF01774016. [DOI] [PubMed] [Google Scholar]
- 9.Huber MB, Carballido-Gamio J, Bauer JS, Baum T, Eckstein F, Lochmüller EM, Majumdar S, Link TM. Proximal femur specimens: automated 3D trabecular bone mineral density analysis at multidetector CT-correlation with biomechanical strength measurement. Radiology. 2008;247(2):472–481. doi: 10.1148/radiol.2472070982. [DOI] [PubMed] [Google Scholar]
- 10.Ito M, Lang TF, Jergas M, Ohki M, Takada M, Nakamura T, Hayashi K, Genant HK. Spinal trabecular bone loss and fracture in American and Japanese women. Calcif Tissue Int. 1997;61(2):123–128. doi: 10.1007/s002239900308. [DOI] [PubMed] [Google Scholar]
- 11.Kang Y, Engelke J, Fuchs S, Kalender WA. Ananatomic coordinate system of the femoral neck for highly reproducible BMD measurements using 3D QCT. Comput Med Imaging Graph. 2005;29(7):533–541. doi: 10.1016/j.compmedimag.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Lafferty FW, Rowland DY. Correlations of dual-energy X-ray absorptiometry, quantitative computed tomography, and single photon absorptiometry with spinal and non-spinal fractures. Osteoporos Int. 1996;6(5):407–415. doi: 10.1007/BF01623015. [DOI] [PubMed] [Google Scholar]
- 13.Lane NE, Sanchez S, Modin GW, Genant HK, Pierini E, Arnaud CD. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. J Clin Invest. 1998;102(8):1627–1633. doi: 10.1172/JCI3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang TF, Keyak JH, Heitz MW, Augat P, Lu Y, Mathur A, Genant HK. Volumetric quantitative computed tomography of the proximal femur: precision and relation to bone strength. Bone. 1997;21(1):101–108. doi: 10.1016/S8756-3282(97)00072-0. [DOI] [PubMed] [Google Scholar]
- 15.Lang TF, Li J, Harris ST, Genant HK. Assessment of vertebral bone mineral density using volumetric quantitative CT. J Comput Assist Tomogr. 1999;23(1):130–137. doi: 10.1097/00004728-199901000-00027. [DOI] [PubMed] [Google Scholar]
- 16.Lang TF, Guglielmi G, van Kuijk C, de Serio A, Cammisa M, Genant HK. Measurement of bone mineral density at the spine and proximal femur by volumetric quantitative computed tomography and dual-energy X-ray absorptiometry in elderly women with and without vertebral fractures. Bone. 2002;30(1):247–250. doi: 10.1016/S8756-3282(01)00647-0. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Sode M, Saeed I, Lang T. Automated registration of hip and spine for longitudinal quantitative CT studies: integration with 3D densitometric and structural analysis. Bone. 2006;38(2):273–279. doi: 10.1016/j.bone.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lian KC, Lang TF, Keyak JH, Modin GW, Rehman Q, Do L, Lane NE. Differences in hip quantitative computed tomography (QCT) measurements of bone mineral density and bone strength between glucocorticoid-treated and glucocorticoid-naïve postmenopausal women. Osteoporosis International. 2005;16(6):642–650. doi: 10.1007/s00198-004-1736-9. [DOI] [PubMed] [Google Scholar]
- 19.Melton LJIII, Cooper C. Magnitude and Impact of Osteoporosis and Fractures. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. 2nd Ed. Vol. 1. San Diego: Academic Press; 2001. pp. 557–567. [DOI] [Google Scholar]
- 20.Wu XP, Dai RC, Shan PF, Yuan LQ, Cao XZ, Liao EY, Jiang Y. Establishment of BMD reference curves at different skeletal sites in women, using a Cartesian coordinate numeration system. Osteoporos Int. 2005;16(12):1655–1662. doi: 10.1007/s00198-005-1898-0. [DOI] [PubMed] [Google Scholar]