Abstract
Objective: To investigate gender difference in the effects of daytime sleep on item and source memories, which are dissociable elements of declarative memory, and the effects of sleep on recollection and familiarity, which are two processes underlying recognition. Methods: Participants saw a series of pictures with either blue or red background, and were then given a pretest for item and source memories. Then males and females respectively were randomly assigned either to a wake or a sleep condition. In the wake condition, participants remained awake until the posttest; in the sleep condition, participants slept for 1 h until awakened and asked to remain awake until the posttest. Results: Daytime sleep contributed to retention of source memory rather than item memory in females, whereas males undergoing daytime sleep had a trend towards increased familiarity. For females, however, neither recollection nor familiarity appeared to be influenced by daytime sleep. Conclusion: The mechanism underlying gender difference may be linked with different memory traces resulting from different encoding strategies, as well as with different electrophysiological changes during daytime sleep.
Keywords: Gender difference, Declarative memory, Recollection, Familiarity, Daytime sleep
INTRODUCTION
Jenkins and Dallenbach (1924) did an experiment demonstrating sleep to be beneficial for memory. Over the past century many other researchers have continued to explore the effect of sleep on memory (Born et al., 2006; Ekstrand, 1967; Yaroush et al., 1971; Walker and Stickgold, 2004; Walker et al., 2002a; Schabus et al., 2004; Gottselig et al., 2004; Drosopoulos et al., 2005; Daurat et al., 2007; Atienza and Cantero, 2008; Rauchs et al., 2008). One reason for such persistent interest is that discovering the effect of sleep on memory has a two-fold significance of contributing to the understanding of both the functions of sleep and mechanisms of human memory.
Declarative memory refers to the memory of facts or events learned through exposure. Although there have been many studies examining the effect of a whole night of sleep, the number of studies investigating the role of a daytime nap on declarative memory is small and the conclusions from them are controversial. In a study by Backhaus and Junghanns (2006), after executing procedural (mirror tracing) and declarative memory tasks (paired associates), participants were assigned to wake and nap conditions that lasted about 45 min, and it was found that the daytime sleep improved procedural memory, rather than declarative memory. Tucker et al.(2006), however, investigated the effect of a period of slow wave sleep (SWS) on procedural and declarative memories (using mirror tracing and paired associates tasks, respectively), and came up with a contrasting conclusion. In this study, following the encoding phase, participants experienced one of the two conditions: they either remained awake (the wake condition) or were allowed a short sleep (the nap condition). In the nap condition, participants were allowed to sleep for about 1 h and, if they obtained SWS during this period, were permitted to continue to sleep until the first period of SWS came to its end. It was found that SWS significantly improved declarative memory, rather than procedural memory. Lahl et al.(2008) conducted two experiments to examine the effect of daytime nap on declarative memory (using a paired associates task). In the first experiment, after the encoding period, participants either remained awake or napped for 1 h. Participants who napped showed better memory performance than those who kept awake. In the second experiment, participants were assigned to one of the three conditions, in which they napped for 1 h, or 6 min, or remained awake for 1 h. The critical finding is that napping, either for 1 h or 6 min, significantly enhanced declarative memory. In addition, participants napping for 1 h demonstrated better memory performance than those napping for only 6 min.
The above mentioned controversy may stem from the fact that both sleep and declarative memory are two complicated variables, between which the relationship can be influenced by a wide variety of factors. Sleep can be broadly categorized as slow wave sleep and rapid eye movement sleep, which may have differential influences on declarative memory. In addition, the different amount of sleep at a specific stage can be a critical factor that accounts for the existent controversy. In the study of Backhaus and Junghanns (2006), the amount of SWS that participants obtained was 8.7 min, while participants in the study of Tucker et al.(2006), however, obtained 22.4 min. The effect of nap can also be modulated by the way in which declarative memory is tested, e.g., recall versus recognition (Diekelmann et al., 2008).
Although there has been evidence supporting the role of sleep in declarative memory, the tasks in many studies do not, in a strict sense, deal with episodic memory, which is an important element of declarative memory. In fact, declarative memory was frequently tested via the task of paired associates. In our opinion, results based on such a task are clearly insufficient for a thorough understanding and an ultimate conclusion of the effect of sleep on declarative memory. In order to have convincing evidence concerning the role of sleep in declarative memory, it is necessary to adopt a task that examines the various elements underlying declarative memory.
Episodic memory is composed of two elements, source and item memories. Source memory refers to recollection or recall of the context from which an item or fact is acquired, whereas item memory refers to recognition or recall of previously presented information itself. To our knowledge, no studies of sleep so far have examined the effects of sleep on source and item memories. There is little doubt that the above two elements of episodic memory are integral to a comprehensive understanding of the effect of sleep on declarative memory. For instance, if it turns out that sleep affects source memory but not item memory, then it is unjustifiable to state generally that sleep has an effect on declarative memory.
Many studies have shown that males and females differ in episodic memory. Guillem and Mograss (2005) found that males and females used different strategies in processing facial pictures. The processing in females entails more detailed elaboration of information content, whereas the processing in males is more likely to be driven by schemas or overall information. Horgan et al.(2004) found that under the conditions of both directed and incidental learning, women recalled information concerning the appearance of their social targets more accurately than men did. Burton et al.(2004) studied gender differences in implicit and explicit memories for affective passages and found that male subjects showed greater priming for affective material than female subjects, and a greater gain in explicit memory for affective material compared to neutral material than female subjects. Piefke et al.(2005) investigated gender differences in functional neuroanatomy of emotional episodic autobiographical memory and found that in males, all types of autobiographical memories investigated were associated with differential activation of the left parahippocampal gyrus. By contrast, the right dorsolateral prefrontal cortex was activated differentially in females. In addition, the right insula was activated differentially in females during remote and negative memory retrieval. These studies demonstrate that gender differences exist in episodic memory.
Thus far, gender differences have been completely ignored in the majority of sleep research. Therefore in this study we aimed to examine whether there would be gender differences in the effect of daytime sleep on declarative memory. In addition, as an extension of the study of Hu et al.(2006), who investigated the effect of nocturnal sleep on memory of emotional pictures, we were interested to know whether R (remember) and K (know) responses as defined by Tulving (1983) would be differentially influenced by daytime sleep. An R response indicates that recollection evokes a specific episode in which the stimulus was previously experienced, and a K response indicates that the recollection is only based on familiarity.
In this study the paradigm of daytime sleep was used because it is less likely to be compromised by the confounding factors, such as circadian rhythm, growth hormone, level of alertness, and cortisol. It was found that during daytime sleep there is hardly any growth hormone secretion and much lower variation in the level of alertness (Monk et al., 1997). Therefore, it can be expected that an investigation that adopts a paradigm of daytime sleep will have more internal validity.
Based on the studies demonstrating gender differences in episodic memory and the studies showing that memory consolidation by sleep is dependent on reactivation and replay of memory traces formed during learning (Pavlides and Winson, 1989; Walker et al., 2002b), we hypothesized that episodic memory of males and females would be differentially influenced by daytime sleep. It was previously discovered that the effect of sleep was subject to modulation of strength of memories and that sleep was favorable to consolidation of memory of weak strength (Stickgold et al., 1999; Walker et al., 2002b). Because K responses are representative of memories of weak strength, we also hypothesized that the accuracy of K responses, which represents familiarity, would be better maintained through daytime sleep compared to that of R responses, which represents recollection. In addition, we expected that males and females would be differently influenced by daytime sleep in terms of recollection and familiarity.
MATERIALS AND METHODS
Participants
Forty participants (20 males and 20 females) aged between 19 and 27 years old (mean=21.54 years, SD=1.79 years) from several universities in Beijing, China, took part in the experiment. All participants were habitual noon sleepers, non-smoking, right-handed, and free from any sleep and mood disorders. They had no history of severe organic or mental illnesses. One week before the experiment they were asked to write sleep diaries. During the experimental days they were required to sleep at 23:00 and get up at 7:00, and were required to refrain from drinking any alcoholic or caffeinated beverages. Each participant was paid 30 yuan for taking part in the experiment.
Design and procedure
Experimental design was shown in Table 1. Twenty male and 20 female participants were respectively randomly assigned to the wake and sleep conditions so that in each condition there were 10 males and 10 females. In the sleep condition, participants arrived at the lab at approximately 13:00, and had electrodes placed on their scalps for 25 min. Then at 13:30 the learning began, in which they memorized a sequence of pictures and their corresponding background colors. The learning took about 5 min. Immediately after learning, they received the pretest that lasted for about 10 min. At 13:50 they were asked to sleep in a bed in a sound-attenuated room. After sleeping for 1 h they were awakened and asked to surf the Internet or play computer games to reduce sleep inertia. At 15:30 they received the posttest, which took about 10 min. The two sets of pictures used prior to and after sleep were counterbalanced.
Table 1.
Experimental design
| Condition | 13:30~13:35 | 13:35~13:45 | 13:50~14:50 | 15:30~15:40 |
| Sleep | Memorize pictures | Pretest | Sleep | Posttest |
| Wake | Memorize pictures | Pretest | Stay awake | Posttest |
In the wake condition, participants reported to the lab at 13:00, and waited until 13:30 when the learning started. Immediately after the learning they received the pretest, and were then required to stay in the lab surfing the Internet or playing computer games until 15:30, when they received the posttest in the same procedure as the sleeping participants experienced. The two sets of pictures used prior to and after the waking period were counterbalanced.
Memory task
During the learning phase, participants were randomly presented with a sequence of 28 pictures, which were originally selected from the picture database by Snodgrass and Vanderwart (1980) and were then standardized in terms of complexity, imagery, and familiarity. Two pictures at the beginning and two pictures at the end of the sequence were used to buffer primacy and recency effects. Constituting the remaining stimuli were 24 pictures of objects that fall into six categories: animals, fruits, clothes, plants, daily utensils, and furniture. In each category, two pictures were displayed upon a background color of blue and two pictures were displayed upon a background color of red. This approach aimed to eliminate the possibility that participants memorized the background color of stimuli simply by memorizing the category to which an object belonged. During each trial, a crosshair first appeared at the center of screen for 500 ms, and then a picture was presented for 4000 ms. Participants sat in front of a computer with a viewing distance of about 50 cm, and were encouraged to use association or any other mnemonics to commit effectively to memory both the pictures and the screen background colors in which they appeared. The learning phase lasted about 5 min.
During the pretest phase, which took place immediately after the learning phase, participants were presented with 24 pictures, half of which were completely new and half selected from the 24 pictures that had been learned. The two sets of pictures were matched in complexity, imagery, and familiarity. For each picture, participants were asked to make one of the following three responses by clicking the mouse as fast and accurately as possible: (1) I remember this picture (R response); (2) I know this picture (K response); (3) I did not see this picture. An R response indicates that participants are able to consciously recollect the context and details associated with the initial presentation of a picture, and a K response indicates that the recollection is only based on familiarity. If they made the first or second choice, they were required to choose the background color in which a specific picture was initially presented during the learning phase. They were asked to click either “red” or “blue” as quickly and accurately as possible. The next picture did not appear until participants made all relevant responses for the current picture. If they made the third choice, then they were presented with the next picture, for which they had to make one of the above three choices again.
During the posttest phase, which occurred 2 h after the initial learning phase, the remaining 12 pictures that had been learned were mixed with another 12 new pictures. As in the pretest phase, the two sets of pictures were matched in terms of complexity, imagery, and familiarity. Participants were randomly presented with the 24 pictures and for each picture they made relevant responses for a picture in the same procedure as they did in the pretest phase.
Sleepiness scale
Both before the learning phase and posttest phase, participants were asked to make a choice on Stanford Sleepiness Scale (http://www.stanford.edu/~dement/sss.html).
Electroencephalogram (EEG) recording
Participants in the sleep condition were monitored continuously with digital EEG acquisition software (NeuroScan, El Paso, TX, USA) using a seven-channel montage: four EEG (C3, C4, O1, O2), two electrooculography [vertical electrooculogram (vEOG) and horizontal electrooculogram (hEOG)], and chin electromyography (EMG), according to the International 10-20 System. C3 and O1 were referenced to M2 (the right mastoid); C4 and O2 were referenced to M1 (the left mastoid). vEOG was recorded bipolarly using two electrodes affixed above and below the left pupil. hEOG was recorded bipolarly from identical electrodes and attached to the outer canthi of both eyes. All signals were filtered (0.10-Hz high-pass filter; 70-Hz low-pass filter; 50-Hz notch filter) and digitized online with a 250-Hz sampling rate. Following artifact rejection for the recorded signals, sleep stages were visually inspected using the standard criteria (Rechtschaffen and Kales, 1968).
Data analysis
Data analyses were conducted using SPSS for Windows 15.0. The level of significance was set to be 0.05. Univariate analyses were made, with the fixed factors being gender (male versus female) and condition (sleep versus wake). The dependent measures of memory improvement were obtained by subtracting pretest accuracy of memory from posttest accuracy of memory. Accuracy of item memory was determined according to the two-high threshold model (Snodgrass and Corwin, 1988). Accuracy of source memory was calculated as the percentage of correctly recalled background colors for the pictures that were correctly judged as having been presented in the learning phase.
Univariate analyses were conducted to analyze the effect of sleep on recollection and familiarity, with the fixed factors being gender (male versus female) and condition (sleep versus wake). The indexes of recollection and familiarity were the proportion of R responses (R hit rates minus R false alarm rates) and the proportion of K responses (K hit rates minus K false alarm rates), respectively. The dependent measures were obtained by subtracting the proportions of R and K responses at the pretest from the corresponding proportions at the posttest. The data of six participants, whose hit rates were lower than false alarm rates in terms of R responses, were excluded. The data of 11 participants, whose hit rates were lower than false alarm rates in K responses, were excluded.
RESULTS
Sleepiness scale
Univariate analyses showed that before the learning there was no significant difference in sleepiness for both the wake and sleep groups. At the posttest, however, participants in the sleep group were significantly less sleepy than those in the wake group [F(1, 33)=8.115, P=0.008]1. Further analyses showed that, both before the learning and at the posttest, male participants in sleep condition did not significantly differ from those in the wake condition. Before the learning, female participants in the sleep condition did not differ from those in the wake condition, but at posttest the former were significantly less sleepy than the latter [F(1, 16)=5.631, P=0.031].
Sleep parameters
The sleep parameters are displayed in Table 2. It can be seen that overall participants needed about 10 min to fall asleep and the total sleep time was more than 30 min. No participant entered rapid eye movement (REM) sleep.
Table 2.
Sleep parameters for all participants in the nap condition
| Sleep parameter | Time (min) |
||
| Males | Females | All participants | |
| SL | 11.25±2.30 | 12.90±1.46 | 12.08±1.34 |
| S1 | 9.81±2.68 | 12.32±2.41 | 11.07±1.77 |
| S2 | 12.91±1.85 | 9.22±1.86 | 11.07±1.35 |
| SWS | 12.77±3.57 | 10.75±4.32 | 11.76±2.74 |
| TST | 35.50±5.23 | 32.30±4.42 | 33.90±3.35 |
All data expressed as mean±SEM. SL: sleep latency; S1: sleep stage 1; S2: sleep stage 2; SWS: slow wave sleep; TST: total sleep time
Effects on item and source memories
Descriptive data for the pretest item and source memory performance were presented in Table 3. It was shown that there was no significant difference in pretest item memory between males and females [F(1, 37)=1.884, P=0.178]. However, there was a trend towards significance in the difference in pretest source memory between males and females [F(1, 37)=3.899, P=0.056].
Table 3.
Accuracies of item and source memories at the pretest for all males and females
| Gender | Accuracy |
|
| Item memory | Source memory | |
| Males | 0.72±0.04 | 0.68±0.04 |
| Females | 0.79±0.03 | 0.79±0.03 |
All data expressed as mean±SEM
Descriptive data for item and source memories both at the pretest and the posttest were given in Tables 4 and 5. Analyses on item memory improvement showed that there were no significant main effects for either gender [F(1, 35)=0.531, P=0.471] and condition [F(1, 35)=0.044, P=0.836]. In addition, there was no significant interaction between gender and condition [F(1, 35)=0.745, P=0.394].
Table 4.
Accuracy for item memory at the pretest and posttest
| Gender | Accuracy |
|||
| Wake |
Sleep |
|||
| Pretest | Posttest | Pretest | Posttest | |
| Males | 0.70±0.05 | 0.65±0.06 | 0.74±0.02 | 0.62±0.06 |
| Females | 0.78±0.04 | 0.63±0.04 | 0.79±0.04 | 0.68±0.05 |
All data expressed as mean±SEM
Table 5.
Accuracy for source memory at the pretest and posttest
| Gender | Accuracy |
|||
| Wake |
Sleep |
|||
| Pretest | Posttest | Pretest | Posttest | |
| Males | 0.66±0.04 | 0.63±0.03 | 0.71±0.07 | 0.48±0.06 |
| Females | 0.81±0.04 | 0.42±0.06 | 0.76±0.05 | 0.63±0.06 |
All data expressed as mean±SEM
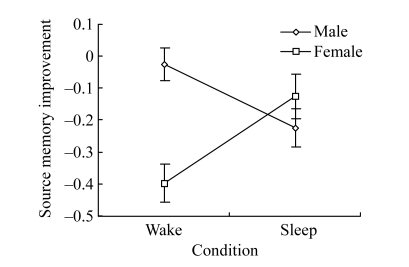
Analyses on source memory improvement showed no significant main effect for either wake or sleep condition. However, there was a significant main effect of gender [F(1, 35)=5.372, P=0.026], indicating that memory improvement of females was significantly smaller than that of males. Interestingly, there was a significant interaction between gender and condition (Fig.1) [F(1, 35)=16.121, P<0.001]. Further analyses demonstrated that, for males, memory improvement under the sleep condition was significantly smaller than that under the wake condition [F(1, 35)=5.92, P=0.02], whereas for females, memory improvement under the sleep condition was significantly greater than that under the wake condition [F(1, 35)=9.92, P=0.003]. Univariate analyses showed that, with regard to pretest score of source memory, participants in the wake condition did not significantly differ from those in the sleep condition, with F(1, 17)=0.763, P=0.395 and F(1, 18)=0.379, P=0.546 for females and males under the two conditions, respectively.
Fig.1.
Gender differences in the effect of sleep on source memory improvement
After sleep, males show a significant decrease in source memory (P=0.02), whereas females show a significant increase in source memory (P=0.003)
Effects on recollection and familiarity
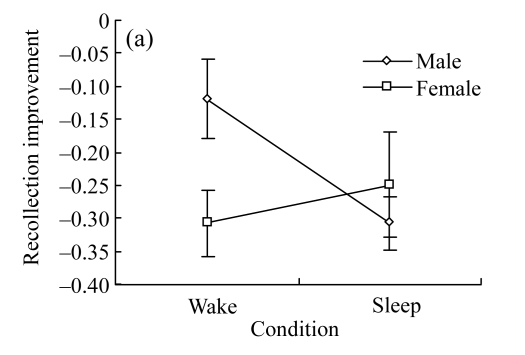
Descriptive data for recollection and familiarity both at the pretest and the posttest were given in Table 6. The changes in recollection and familiarity relative to the pretest were given in Table 7. As to the analyses on recollection improvement, no significant main effect was found either for gender [F(1, 30)=1.202, P=0.282] or for condition [F(1, 30)=1.202, P=0.282]. However, there was a significant interaction between gender and condition (Fig.2a) [F(1, 30)=4.273, P=0.047]. Further analyses indicated that for males, recollection under the sleep condition was significantly less than that under the wake condition [F(1, 30)=5.22, P=0.03]. However, for females, condition had no significant effect on recollection [F(1, 30)=0.54, P=0.47].
Table 6.
Values of recollection (R) and familiarity (F) at pretest and posttest
| Gender | Wake |
Sleep |
||||||
| Pretest |
Posttest |
Pretest |
Posttest |
|||||
| R | F | R | F | R | F | R | F | |
| Males | 0.06±0.07 | 0.18±0.07 | 0.52±0.06 | 0.18±0.07 | 0.68±0.04 | 0.11±0.03 | 0.38±0.04 | 0.26±0.03 |
| Females | 0.63±0.06 | 0.14±0.03 | 0.32±0.06 | 0.28±0.06 | 0.58±0.07 | 0.30±0.07 | 0.33±0.04 | 0.40±0.05 |
All data expressed as mean±SEM. R and F are calculated on the basis of accuracies of R and K responses
Table 7.
Improvement in recollection and familiarity (relative to pretest performance)
| Gender | Wake |
Sleep |
||
| R improvement | F improvement | R improvement | F improvement | |
| Males | −0.11±0.19 | 0.00±0.12 | −0.31±0.04 | 0.15±0.04 |
| Females | −0.31±0.05 | 0.13±0.05 | −0.25±0.07 | 0.10±0.05 |
All data expressed as mean±SEM
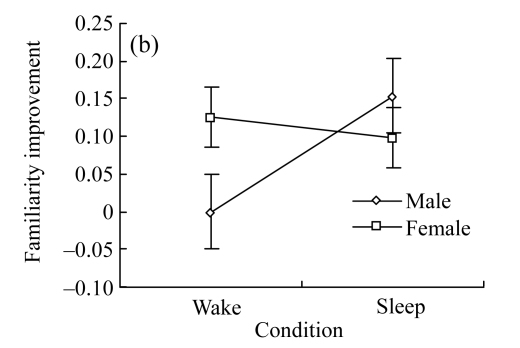
Fig.2.
Gender differences in the effect of daytime sleep on recollective states
(a) Gender differences in the effect of sleep on recollection improvement. After sleep, males have significantly less recollection under sleep condition than under wake condition (P=0.03), whereas females are not influenced by sleep; (b) Gender differences in the effect of sleep on familiarity improvement. After sleep, males have marginally significantly more familiarity (P=0.051), whereas females show no significant difference in familiarity
In terms of analyses on familiarity, no significant main effect was found either for gender [F(1, 25)=0.479, P=0.495] or for condition [F(1, 25)=1.594, P=0.218]. There was, however, marginally significant interaction between gender and condition (Fig.2b) [F(1, 25)=3.163, P=0.087]. Further analyses showed that, for males, familiarity improvement under the sleep condition was marginally significantly greater than that under the wake condition [F(1, 25)=4.18, P=0.051], whereas familiarity of females was not influenced by condition [F(1, 25)=0.16, P=0.689].
Correlations between sleep parameters and memory performance
Pearson’s correlational analyses based on data from all participants in the nap condition showed that neither item memory improvement nor source memory improvement was significantly correlated with any of the sleep parameters (P>0.05 in both groups). However, memory performance at the posttest was significantly correlated with the duration of sleep stage 1 (for item memory, r=0.45, P=0.046; for source memory, r=0.487, P=0.03). In addition, item memory at the posttest was marginally significantly correlated with total sleep time (r=0.39, P=0.089); source memory at the posttest was significantly correlated with total sleep time (r=0.486, P=0.03).
Analyses based solely on data of all male participants in the nap condition showed that neither item memory improvement nor source memory improvement was significantly correlated with any of the sleep parameters (P>0.05). However, analyses based solely on data of all female participants in the nap condition showed that item memory improvement was significantly correlated with the duration of sleep stage 2 (r=−0.634, P=0.049), and source memory improvement was marginally significantly correlated with the duration of sleep stages 1 (r=0.578, P=0.08) and 2 (r=−0.598, P=0.068).
In analyzing the correlation between recollection and familiarity improvements and sleep parameters, for males we did not find any significant correlation. However, for females recollection improvement was significantly correlated with the duration of sleep stage 2 (r=−0.758, P=0.029), and familiarity improvement was marginally significantly with total sleep time (r=0.732, P=0.062).
DISCUSSION
Effects on item and source memories
To the best of our knowledge, this is the first research into gender differences in the effects of daytime sleep on item and source memories. Our study mainly aimed to answer two questions: (1) Does daytime sleep have different effects on item and source memory performance for males and females? (2) Does daytime sleep have different effects on recollection and familiarity for males and females?
Compared to previous studies showing the effect of sleep on declarative memory, our investigation has yielded several new findings: (1) 1 h of daytime sleep does not have an effect on item memory, but does have an effect on source memory. (2) The effect of 1 h of daytime sleep interacts with gender of participants; that is, such a period of sleep contributes to source memory for females, but is not beneficial for males. (3) It is males, rather than females, whose recollection and familiarity tend to be influenced by sleep, for napping males had significantly less recollection but numerically more familiarity.
Although it is very well-established that sleep has an effect on procedural memory, the debate continues as to whether sleep can also influence declarative memory. The findings of our study are consistent with the growing body of evidence suggesting the role of sleep in declarative memory (Tucker et al., 2006; Born et al., 2006; Walker and Stickgold, 2004; Walker et al., 2002b; Schabus et al., 2004; Gottselig et al., 2004; Drosopoulos et al., 2005; Rauchs et al., 2008). However, a new finding from our study is that the effect of sleep varies depending upon the specific elements of declarative memory. Although item memory for pictures seemed immune to the effect of sleep, source memory, which is in our study the memory for background color of pictures, benefited from sleep. Therefore, when considering the role of sleep in declarative memory, it is necessary to be clear about the specific element of declarative memory in the first place. It is risky to make a conclusive remark about a general or universal effect of sleep on declarative memory.
Our study shows that, for females, daytime sleep contributed to source memory but not to item memory. This finding adds to the existing bulk of evidence supporting the dissociation between source and item memories. Our results are consistent with the finding that item and source memories have been observed dependent upon separate neural substrates (Slotnick et al., 2003), and with the mechanism that sleep is a process during which specific brain areas are activated. It might be speculated that the neural structures responsible for processing source memory are preferentially activated in sleep (at least for females).
With regard to source memory, we found that males and females were differentially affected by daytime sleep and females had better source memory retention whereas males had worse source memory retention. It might be hard to explain this phenomenon, but we attempt to provide some speculations. The first reason may be that, during the learning phase, females used mnemonic strategies different from the ones used by males, despite the same instructions provided for both males and females. It is likely that different memory strategies lead to different memory traces. In particular, females have been shown to be superior to males in verbal memory. Females are more likely to employ their verbal advantage even if a memory task requires other forms of mental processing (Herlitz and Rehnman, 2008). In our study, participants were required to memorize both the pictures and the corresponding background colors, and they were encouraged to use mnemonic devices to associate the pictures and the background colors. Compared to males, females are more likely to rely more upon their verbal advantage to establish more hippocampus-dependent memory traces. The comparison analysis based on data in Table 3 provides some support to our speculation that females may use better encoding strategies that enable them to bind more successfully an object to its contextual features, which, in turn, led to greater reactivation during sleep in the hippocampus, where the traces of source memory are stored temporarily and then transferred to cortical areas. Buzsaki (1989) suggests that primarily during SWS a “hippocampo-neocortical dialog” occurs to strengthen the memory trace, with the hippocampus generating spontaneous sharp wave-ripple (SPW-R) complexes, which presumably provide efferent potentiation of cortical targets activated during information encoding.
The second reason may be that, although females do not differ from males in terms of total sleep time, females who experienced a period of sleep may undergo different, subtle electrophysiological changes that are favorable to source memory. Indeed, a study that examined gender differences in sleep spindle topography found that females showed a significantly higher percentage of spindles in the left frontal channel than males (Huupponen et al., 2002). Considering a number of studies showing that SWS and sleep spindles are critical to consolidation of declarative memory (Clemens et al., 2005; 2006; Peigneux et al., 2004; Schabus et al., 2004), it seems reasonable that we only found females to benefit from daytime sleep.
One possible explanation for better source memory performance of the females undergoing sleep is that they were at a higher level of alertness at the posttest. Apparently, such an explanation is inadequate and unsatisfying. Pearson’s correlation analysis showed that for females there was no significant correlation between sleepiness and source memory performance at the posttest (r=−0.054, P=0.831). To further examine the effect of sleepiness on source memory performance, we also did Pearson’s correlation analysis based on the pretest data, and found no significant correlation between sleepiness and source memory performance (r=−0.013, P=0.960). In addition, we did analysis based on the respective change in sleepiness and source memory performance improvement, but we found no significant correlation between the changes in sleepiness (i.e., sleepiness at posttest minus sleepiness at pretest) and source memory improvement (i.e., source memory scores at posttest minus source memory scores at pretest) (r=−0.016, P=0.949). The above analyses demonstrated that sleepiness was not a factor involved in source memory improvement for females.
Another explanation for better source memory retention of napping females is that they had less interference compared to the females in the wake condition. However, this explanation is not satisfying in that item memory was not influenced. In addition, if reduced interference per se can account for the memory performance, then napping males should also have shown better memory performance, which is not what we observed.
It is important to note that the source memory of males actually suffered from 1 h of daytime sleep, indicating that at least in the domain of source memory, there may exist different mechanisms of consolidation for males and females. For males, a period of wakefulness seems to better support consolidation of source memory. Indeed, in one study that used a “tracking” task to evaluate the modulator effects of spatial and procedural learning on brain activation across multiple scanning sessions (Peigneux et al., 2004), it was found that the pattern of brain activation associated with the unrelated task shifted over a 2-h period of repeated scans during which volunteers remained awake. In another study that used auditory identification learning (Roth et al., 2005), it was found that that 12 h post-training in the waking state was as effective as 12 h including no less than 6 h of night’s sleep. This study demonstrated that the existence of a latent, hours-long, consolidation phase in a human auditory verbal learning task could occur even during wakefulness. In fact, despite the growing evidence concerning the contribution of sleep to memory consolidation, it is far from clear that under which conditions sleep is necessary, simply favorable, or unimportant to memory consolidation (Walker, 2005).
Effects on recollection and familiarity
After sleep males had significantly less recollection but tended to have more familiarity, whereas the recollection and familiarity of females were not influenced by sleep. This finding is consistent with the finding of Hu et al.(2006) that sleep facilitated familiarity, but not recollection, of emotional declarative memory. However, our finding is in contrast to the finding of Drosopoulos et al.(2005), who found that sleep enhanced only explicit recollection. The reason for this disagreement might lie in the difference in experimental paradigms; we used a remember/know procedure, but Drosopoulos et al.(2005) used a process dissociation procedure. Another reason might be linked with the difference of materials. In our study, pictures with either red or blue background were used as stimuli, but in the study by Drosopoulos et al.(2005), words were employed as stimuli. It is possible that, although both memories for pictures and words are hippocampus-dependent, these two forms of memories may involve slightly different neural networks. It is difficult, however, to come up with a definite explanation through this behavioral study.
Although no significant main effect of sleep was observed for recognition performance as a whole, our study suggests that sleep does tend to influence one component of recognition: familiarity. However, only the males undergoing sleep, compared to the males undergoing the same interval of wakefulness, tend to show an increase in familiarity but a decrease in recollection. While the exact mechanism underlying the different effects of sleep on recollection and familiarity remains unclear, it may be because recollection and familiarity are dependent upon distinct neural substrates. Using event-related potentials (ERPs), Duarte et al.(2004) found that during retrieval, familiarity was associated with an enhanced positivity at frontopolar scalp sites from 150 to 450 ms, whereas recollection was associated with positive ERP modulations over bilateral frontal (300~600 ms) and parietal (450~800 ms) sites. To explain our results, it might be speculated that for males, the neural substrates respectively responsible for familiarity and recollection are differentially influenced by a period of daytime SWS. However, this is certainly a preliminary notion whose confirmation demands neuroimaging techniques.
Correlations between sleep parameters and memory performance
Many studies have found that sleep parameters are correlated with subsequent memory performance. For example, Nishida and Walker (2007) found that the duration of sleep stage 2 was correlated with motor memory performance. However, to our knowledge, no studies so far have considered gender differences in correlational analyses. Are males and females differentially influenced by specific sleep parameters?
The correlational analyses we made provided some insights to the above question. While for males none of the sleep parameters was correlated with memory performance, a different pattern occurred for females, whose item memory was significantly correlated with sleep stage 2 and whose source memory was marginally significantly correlated with sleep stages 1 and 2. Gender difference also existed as to the correlations between sleep parameters and recollective states. For males, neither recollection nor familiarity was significantly correlated with any of the sleep parameters; however, for females, recollection was significantly correlated with sleep stage 2 and familiarity was marginally significantly correlated with total sleep time. These different correlational patterns between males and females add some support to our speculation that during daytime sleep males and females may undergo different physiological and neurochemical changes. In summary it might be said that females are more likely than males to be subject to the influences of daytime sleep.
Acknowledgments
We thank Qingfang Zhang of the Institute of Psychology of Chinese Academy of Sciences, who provided the pictures rated in complexity, imagery, and familiarity. We are grateful for Ping Gu at the First Hospital of Hebei Medical University for her support in data collection. Thanks also go to Zheng Yan of the State University of New York at Albany, USA, and Judy Fleit of Queensland University of Technology, Australia, who provided very useful suggestions for the initial draft.
Footnotes
Project supported partially by the National Basic Research Program (973) of China (No. 2006CB303101) and the National Natural Science Foundation of China (No. 90820305)
Sleepiness data of three participants were not collected because of technical problems
References
- 1.Atienza M, Cantero JL. Modulatory effects of emotion and sleep on recollection and familiarity. Journal of Sleep Research. 2008;17(3):285–294. doi: 10.1111/j.1365-2869.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 2.Backhaus J, Junghanns K. Daytime sleeps improve procedural motor memory. Sleep Medicine. 2006;7(6):508–512. doi: 10.1016/j.sleep.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Born J, Rasch J, Gais S. Sleep to remember. The Neuroscientist. 2006;12(5):410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- 4.Burton LA, Rabin L, Vardy SB, Frohlich J, Wyatt G, Dimitri D, Constante S, Guterman E. Gender differences in implicit and explicit memory for affective passages. Brain and Cognition. 2004;54(3):218–224. doi: 10.1016/j.bandc.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Buzsaki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31(3):551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 6.Clemens Z, Fabó D, Halász P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132(2):529–535. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Clemens Z, Fabó D, Halász P. Twenty-four hours retention of visuospatial memory correlates with the number of parietal sleep spindles. Neuroscience Letters. 2006;403(1-2):52–56. doi: 10.1016/j.neulet.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 8.Daurat A, Terrier P, Foret J, Tiberge M. Slow wave sleep and recollection in recognition memory. Consciousness and Cognition. 2007;16(2):445–455. doi: 10.1016/j.concog.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Diekelmann S, Landolt HP, Lahl O, Born J, Wagner U. Sleep loss produces false memories. PLoS ONE. 2008;3(10):e3512. doi: 10.1371/journal.pone.0003512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drosopoulos S, Wagner U, Born J. Sleep enhances explicit recollection in recognition memory. Learning and Memory. 2005;12(1):44–51. doi: 10.1101/lm.83805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duarte A, Ranganath C, Winward L, Hayward D, Knight RT. Dissociable neural correlates for familiarity and recollection during the encoding and retrieval of pictures. Cognitive Brain Research. 2004;18(3):255–272. doi: 10.1016/j.cogbrainres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Ekstrand BR. Effect of sleep on memory. Journal of Experimental Psychology. 1967;75(1):64–72. doi: 10.1037/h0024907. [DOI] [PubMed] [Google Scholar]
- 13.Gottselig GM, Hofer-Tinguely G, Borbély AA, Regel SJ, Landolt HP, Rétey JV, Achermann P. Sleep and rest facilitate auditory learning. Neuroscience. 2004;127(3):557–561. doi: 10.1016/j.neuroscience.2004.05.053. [DOI] [PubMed] [Google Scholar]
- 14.Guillem F, Mograss M. Gender differences in memory processing: evidence from event-related potentials to faces. Brain and Cognition. 2005;57(1):84–92. doi: 10.1016/j.bandc.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Herlitz A, Rehnman J. Sex differences in episodic memory. Current Directions in Psychological Science. 2008;17(1):52–56. doi: 10.1111/j.1467-8721.2008.00547.x. [DOI] [Google Scholar]
- 16.Horgan TG, Mast MS, Hall JA, Carter JD. Gender differences in memory for the appearance of others. Personality and Social Psychology Bulletin. 2004;30(2):185–196. doi: 10.1177/0146167203259928. [DOI] [PubMed] [Google Scholar]
- 17.Hu P, Stylos-Allan M, Walker MP. Sleep facilitates consolidation of emotional declarative memory. Psychological Science. 2006;17(10):891–898. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- 18.Huupponen E, Himanen SL, Värri A, Hasan J, Lehtokangas M, Saarinen J. A study on gender and age differences in sleep spindles. Neuropsychobiology. 2002;45(2):99–105. doi: 10.1159/000048684. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins JG, Dallenbach KM. Oblivicence during sleep and waking. American Journal of Psychology. 1924;35(4):605–612. doi: 10.2307/1414040. [DOI] [Google Scholar]
- 20.Lahl O, Wispel C, Willigens B, Pietrowsky R. An ultra short episode of sleep is sufficient to promote declarative memory performance. Journal of Sleep Research. 2008;17(1):3–10. doi: 10.1111/j.1365-2869.2008.00622.x. [DOI] [PubMed] [Google Scholar]
- 21.Monk TH, Buysse DJ, Reynolds CF3rd, Berga SL, Jarrett DB, Begley AE, Kupfer DJ. Circadian rhythms in human performance and mood under constant conditions. Journal of Sleep Research. 1997;6(1):9–18. doi: 10.1046/j.1365-2869.1997.00023.x. [DOI] [PubMed] [Google Scholar]
- 22.Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE. 2007;2(4):e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavlides C, Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. Journal of Neuroscience. 1989;9:2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, Phillips C, Degueldre C, Del Fiore G, Aerts J, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44(3):535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Piefke M, Weiss PH, Markowitsch HJ, Fink GR. Gender differences in the functional neuroanatomy of emotional episodic autobiographical memory. Human Brain Mapping. 2005;24(4):313–324. doi: 10.1002/hbm.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rauchs G, Orban P, Schmidt C, Albouy G, Balteau E, Degueldre C, Schnackers C, Sterpenich V, Tinguely G, Luxen A, et al. Sleep modulates the neural substrates of both spatial and contextual memory consolidation. PLoS ONE. 2008;3(8):e2949. doi: 10.1371/journal.pone.0002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rechtschaffen A, Kales A. A Manual for Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, DC: U.S. Government Printing Office; 1968. [Google Scholar]
- 28.Roth DAE, Kishon-Rabin L, Hildesheimer M, Karni A. A latent consolidation phase in auditory identification learning: time in the awake state is sufficient. Learning and Memory. 2005;12(2):159–164. doi: 10.1101/87505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schabus M, Gruber G, Parapatics S, Sauter C, Klösch G, Anderer P, Klimesch W, Saletu B, Zeitlhofer J. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27:1479–1485. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 30.Slotnick SD, Moo LR, Segal JB, Hart JJr. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research. 2003;17(1):75–82. doi: 10.1016/S0926-6410(03)00082-X. [DOI] [PubMed] [Google Scholar]
- 31.Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity and visual complexity. Journal of Experimental Psychology (Human Learning) 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- 32.Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. Journal of Experimental Psychology General. 1988;117(1):34–50. doi: 10.1037/0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- 33.Stickgold R, Scott L, Rittenhouse C, Hobson JA. Sleep-induced changes in associative memory. Journal of Cognitive Neuroscience. 1999;11(2):182–193. doi: 10.1162/089892999563319. [DOI] [PubMed] [Google Scholar]
- 34.Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. A daytime sleep containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiology of Learning and Memory. 2006;86(2):241–247. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Tulving E. Elements of Episodic Memory. New York, USA: Oxford University Press; 1983. [Google Scholar]
- 36.Walker MP. A refined model of sleep and the time course of memory formation. Behavorial and Brain Science. 2005;28(1):51–64. doi: 10.1017/s0140525x05000026. [DOI] [PubMed] [Google Scholar]
- 37.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44(1):121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 38.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35(1):205–211. doi: 10.1016/S0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 39.Walker MP, Liston C, Hobson JA, Stickgold R. Cognitive flexibility across the sleep-wake cycle: REM-sleep enhancement of anagram problem solving. Cognitive Brain Research. 2002;14(3):317–324. doi: 10.1016/S0926-6410(02)00134-9. [DOI] [PubMed] [Google Scholar]
- 40.Yaroush R, Sullivan MJ, Ekstrand BR. Effect of sleep on memory. II. Differential effect of the first and second half of the night. Journal of Experimental Psychology. 1971;88(3):361–366. doi: 10.1037/h0030914. [DOI] [PubMed] [Google Scholar]