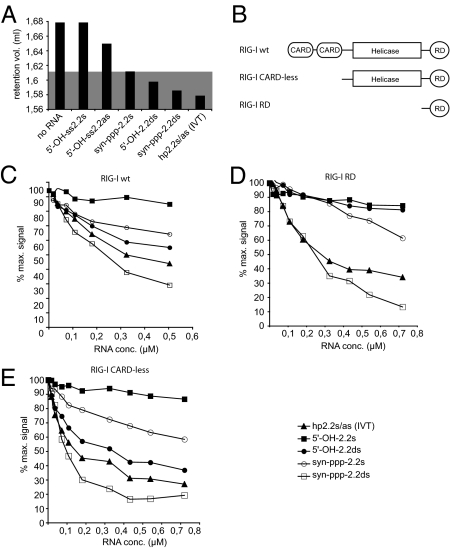
Fig. 4.
Double-stranded 5′-triphosphate RNAs have multiple binding sites on RIG-I and induce dimerization of RIG-I. (A) Different RNAs were examined for their ability to induce homodimerization of recombinant full-length RIG-I protein. The protein-RNA mixture was loaded onto a gel filtration column, and the absorbance at 280 nm was determined. RIG-I elution volume peaks of the different mixtures are represented as columns. The gray area indicates the range of values interpreted as dimeric RIG-I-RNA complexes. (B) Schematic representation of the recombinant RIG-I proteins used in C, D, and E. (C) Recombinant RIG-I full-length protein prebound to fluorescently labeled 5′-ppp-hp2.2s/as RNA (IVT) was incubated with increasing amounts of unlabeled competitor RNAs. Fluorescence anisotropy was determined multiple times after each addition and equilibration. Binding and competition of the unlabeled RNAs is seen as a progressive loss of anisotropy signal. Background values were subtracted, and the anisotropy value of labeled RNA bound to RIG-I in the absence of a competitor was set as 100%. (D) The RNAs used in C were assayed by using recombinant RIG-I RD domain. (E) The RNAs used in C were assayed by using recombinant RIG-I protein lacking both CARD domains.