Figure 5.
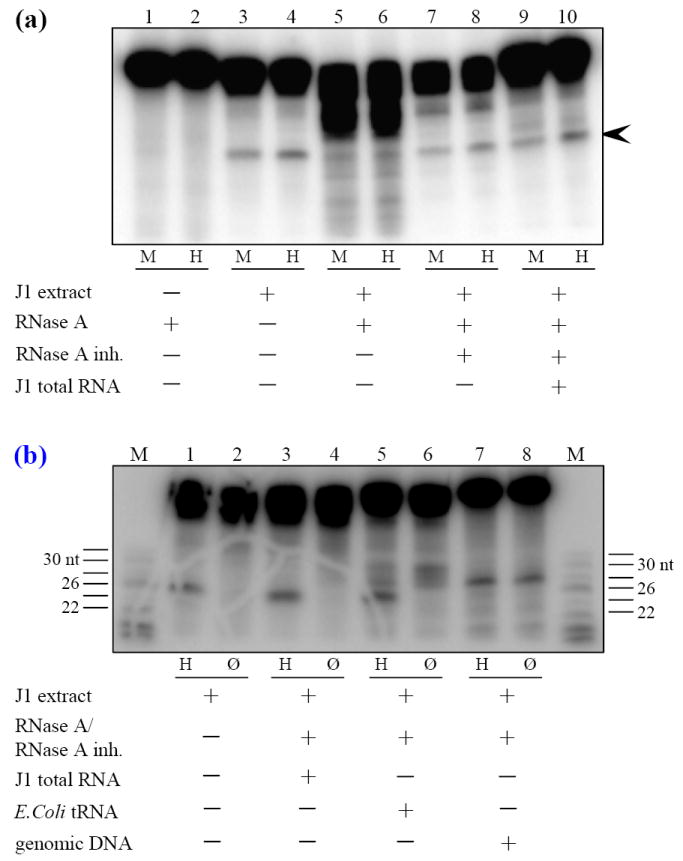
Evidence for the existence of an endogenous RNA component essential to T·G mismatch repair. (a) T·GC ODN was treated with RNase A (Lanes 1, 2), J1 nuclear extract (Lanes 3, 4) or aliquots of a J1 nuclear extract were treated with DNase-free RNase A for 30 min (Lanes 5, 6), with RNase A blocked with an excess of RNase A inhibitor (Lanes 7, 8), or treated with RNase A for 30 min followed by addition of RNase A inhibitor for 30 min and then supplemented with 10 μg of J1 total RNA (Lanes 9, 10). The untreated or treated aliquots were used in T·GC repair assays. The expected 26 nt repair product is indicated by the arrowhead. Both the untreated extract and the extract treated with blocked RNase A were competent for repair. The extract treated with RNase A alone was not able to repair the ODN. However, when extracts treated with RNase A followed by RNase A inhibitor were supplemented with J1 total RNA (lanes 9, 10), they regained the ability to correctly repair the T·GC ODN. Naked ODN incubated with RNase A for 16 hs at 37°C was not detectably degraded (lanes 1, 2). M, MspI. H, HpaII. (b) J1 nuclear extracts treated sequentially with RNase A and RNase inhibitor were supplemented with J1 total RNA, E. coli tRNA or RNase-treated salmon sperm genomic DNA as indicated. Reactions were split in half and either digested with HpaII or not digested. Addition of tRNA or genomic DNA resulted in non-specific cleavage of the ODN phosphodiester backbone (lanes 6, 8 respectively). M, sizing markers. H, HpaII. Ø, no digestion.
