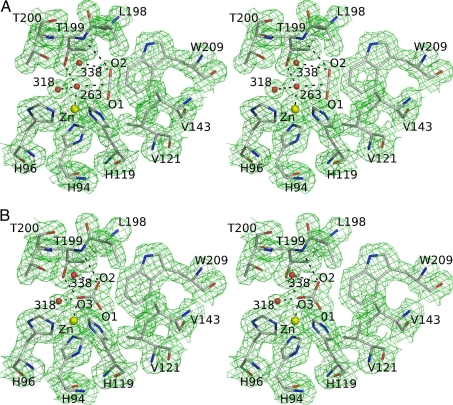
Fig. 2.
Stereoview of HCAII in complex with carbon dioxide and bicarbonate. Distances listed below are indicated in the figures by dashed lines. (A) The 1σ level 2|Fo| − |Fc| electron density map and corresponding model for the CO2-loaded HCAII active site at 1.56-Å resolution. The carbon dioxide O2 atom is at a hydrogen-bonding distance of 3.4 Å from the main chain nitrogen of Thr-199, Wat-338 (3.1 Å), and Wat-263 (3.1 Å). The CO2 molecule also makes van der Waals contacts with all residues lining the hydrophobic pocket (Val-121, Val-143, Leu-198, and Trp-209) and the zinc ligands His-94 and His-119. (B) The 1σ level 2|Fo| – |Fc| electron density maps and the corresponding model for the complex of HCAII and bicarbonate at 1.66-Å resolution. The O3 oxygen of bicarbonate is bound to the zinc ion at 2.0 Å and is within hydrogen-bonding distance of Wat-318 (2.4 Å), Thr-199 OG1 (2.6 Å), and Wat-338 (2.9 Å). The bicarbonate O2 atom is within hydrogen-bonding distance of Wat-338 (2.7 Å) and Thr-199 N (3.1 Å). The O1 atom of bicarbonate is 2.9 Å away from the zinc ion. The HCO3− has van der Waals contacts with the same residues as CO2 (see A).