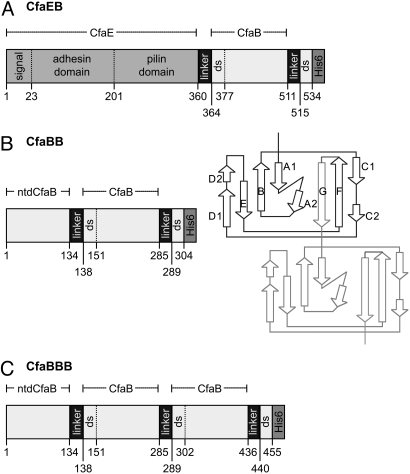
Fig. 1.
Schematic depiction of recombinant fusion proteins containing CfaB. (A) CfaEB comprises (in sequence from N to C terminus) native full-length CfaE, including the N-terminal signal peptide, adhesin domain, and pilin domain; a tetrapeptide linker (designated hereafter as linker); mature CfaB (i.e., without signal peptide); linker; the first 19 aa of a mature CfaB (containing the donor strand, ds); and a hexahistidine affinity tag (His)6. (B) CfaBB comprises mature CfaB with an N-terminal deletion (ntd) of its first 15 residues; linker; a copy of mature CfaB; linker; the first 15 residues from the mature CfaB N terminus (containing the ds); (His)6. The diagram illustrates how this genetic construct replicates the donor-strand exchange mechanism of assembled CFA/I fimbriae. In the native fimbria, 1 CfaB subunit (dark lines) has a hydrophobic groove filled by the N-terminal extension of the next subunit (light lines). In our construct, this connection is enforced by addition of a 4-residue linker between strand F of the first CfaB subunit and strand G of the second subunit. (C) CfaBBB comprises CfaBB with an additional copy of the linker and mature CfaB, as shown. The starting residue number of each segment is denoted along the bottom of each protein depiction, whereas the extent of CfaE, ntdCfaB, and mature CfaB are shown above each depiction.