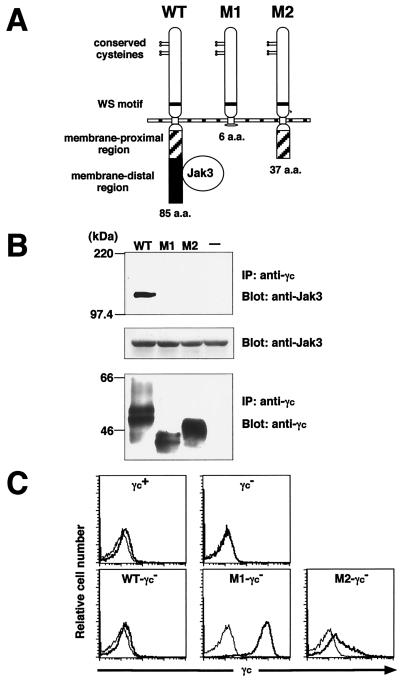
Figure 1.
Generation of mice expressing the mutant γc cDNA on a γc null background. (A) Schematic view of the murine γc chain and its mutants. WT, M1-, and M2-mutant γc retain 85, 6, and 37 aa of the cytoplasmic domain, respectively. The M2-mutant lacks the carboxyl-terminal 48 aa essential for Jak3 binding. (B) Absence of Jak3 association with the M2-mutant. COS cells were transfected with Jak3 and the indicated cDNA by using Lipofectamine Plus reagents (GIBCO/BRL). The cell lysates were immunoprecipitated (IP) with anti-γc (mixture of antibodies derived from 4G3 and TUGm3 clones, PharMingen). Immunoprecipitates were separated by 7.5% SDS/PAGE and subsequently immunoblotted (Blot) with anti-Jak3 (06–342, Upstate Biotechnology) (Top) or anti-γc antibody (sc-669, Santa Cruz Biotechnology) (Bottom). Whole cell lysates were separated by 7.5% SDS/PAGE and immunoblotted with anti-Jak3 antibody to determine the Jak3 protein levels (Middle). (C) Expression of γc on thymocytes. Thymocytes from 4-week-old Tg-γc− mice were stained with mAbs against γc, CD4, and CD8. Data were gated on CD4+ CD8+ cells. Profiles for unstained (fine line) and anti-γc-stained (bold line) thymocytes are shown in the histograms.