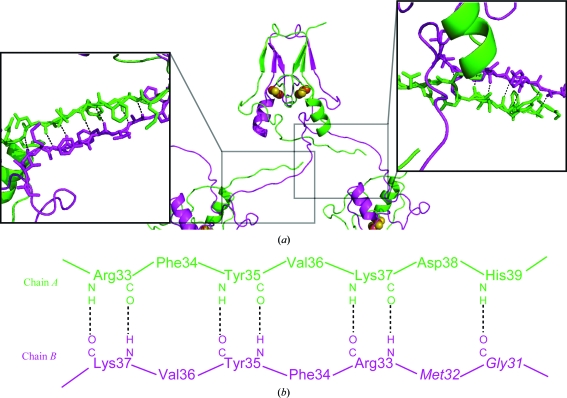
Figure 2.
N-terminal crystal-packing interactions of mitoNEET purified from the sfGFP-mitoNEET fusion construct. (a) The crystal structure of mitoNEET purified from the sfGFP-fusion construct and part of two symmetry-related molecules (center panel) that interact through the N-termini (right and left boxes). The structure of mitoNEET was determined to 1.4 Å resolution. The 2Fe–2S centers are shown as atomic spheres (Fe in red and S in yellow). As with other mitoNEET structures, the two 2Fe–2S domains are within 16 Å (center to center) and are related by a dyad axis. In contrast to previous structures, the extended N-termini induced novel crystal contacts within the crystal. These interactions each involve seven backbone hydrogen bonds (see boxes) that are unique to each terminus of the two protomers. (b) The amino-acid residues in the N-terminal regions that interact through backbone hydrogen bonds (dotted lines) in the crystal structure. The different conformations of the two N-termini result from a ‘register’ shift of the backbone interactions from Arg33 through His39 in chain A and Gly31 through Lys37 in chain B.