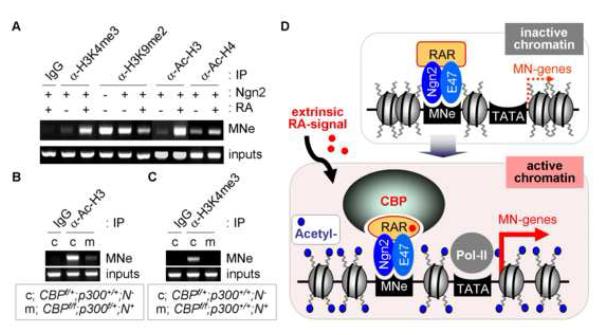
Figure 8. Transcriptionally active chromatin is established by RA and CBP in MNe.
(A) The histone modifications in MNe upon RA treatment were analyzed by ChIP assays in P19 cells expressing Ngn2. RA facilitates histone H3/H4-acetylation and H3-lysine-4-trimethylation (H3K4me3), while suppressing H3-lysine-9-diemthylation (H3K9me2), in MNe. (B, C) ChIP assays using the spinal cord dissected from mutant embryos of genotypes shown in the boxes. Histone H3-acetylation (B) and H3K4me3 (C) in MNe are impaired in CBP-inactivated E12.5 embryonic spinal cord. (D) The working model. Ngn2 and RAR form a complex in pMN progenitors. The extrinsic signal RA binds RAR and facilitates the association of a chromatin modifier CBP with the Ngn2/RAR-complex. Assembly of the Ngn2/RAR/CBP-complex on Ngn2-target motor neuron enhancers triggers their transcriptionally active open chromatin structure marked by H3/4-acetylation and results in subsequent motor neuron gene expressions, leading to the differentiation to motor neurons.