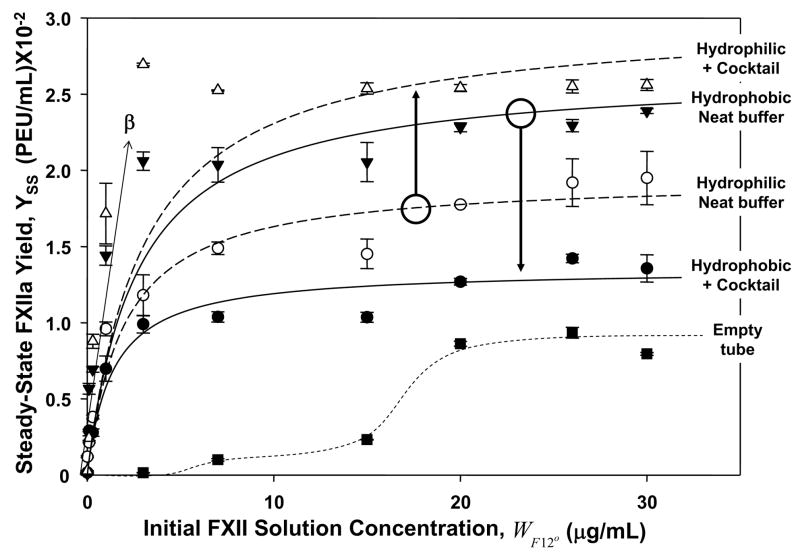
Figure 2.
Steady-state activation of FXII in neat-buffer and protein-cocktail solutions measuring FXIIa solution yield YSS (expressed in plasma-equivalent-units-per-milliliter, PEU/mL) after 30 min. contact with hydrophilic (fully water-wettable glass) procoagulant particles or hydrophobic (poorly water wettable, silanized) procoagulant particles (each at 500 mm2 nominal surface area). Annotations identify procoagulant surface type in either neat-buffer (open circles and filled inverse triangles) or protein-cocktail solution (+ cocktail; filled circles and open inverse triangles). Lines through the neat-buffer and protein-cocktail data represent the best fit to the mathematical model of FXII activation discussed in the text and derived in Appendix I. Curve marked “Empty tube” (filled squares) is a kind of negative control without procoagulant particles measuring FXII activation by contact with the polystyrene tubes used in the assay (dashed curve through the data is a guide to the eye). Initial, linear-like dependence of Yss with FXII solution concentration (μg/mL) provides a measure of the parameter β used to convert YSS in PEU/mL to FXIIa w/v solution concentration WF12a in mg/mL. Note that YSS produced by contact with hydrophilic procoagulants is significantly higher in protein-cocktail solution than neat buffer. In sharp contrast, YSS produced by contact with hydrophobic procoagulants is significantly lower in protein-cocktail solution than neat buffer.